Electrolysis | O Level Chemistry 5070 & IGCSE Chemistry 0620 | Detailed Free Notes
Topics:
- Electrolysis
- Conductor, Non-Conductor and Insulators
- Electrolytes
- Reactivity Series
- Anode and Cathode
- Electrolysis of molten Sodium Chloride
- Electrolysis of aqueous Sodium Chloride
- Electrolysis of dilute Sodium Chloride
- Electrolysis of conc. Sulfuric acid
- Electrolysis of conc. Hydrochloric acid
- Factors affecting Electrolysis
- Applications of Electrolysis
- Production of Aluminum
Electrolysis:
Decomposition of a liquid (molten or aqueous) by the passage of an electric current.
Conductor, non-conductor and insulator:
Conductor:
A conductor is a solid substance which conducts electricity but is not chemically changed during the conduction e.g. metals and graphite.
Non-Conductor:
A non-conductor is a solid substance that does not allow electricity to pass through e.g. salt.
Insulator:
Non-conductors that act as a protection against electric shocks are known as insulators e.g. plastic.
Electrolytes:
Electrolyte:
Electrolytes are compounds which, when molten or dissolved in water, conduct electric current and are decomposed in the process.
Non-electrolyte:
A covalent liquid that does not conduct electricity.
Wea Electrolyte:
A liquid which does not fully ionize and undergoes electrolysis.
Strong Electrolyte:
A liquid which is fully ionized and undergoes electrolysis.
Reactivity Series:
- For positive ions, the reactivity depends on the ease of discharge. As we go down the series, ease of discharge increases and hence, the reactivity decreases.
- For negatively charged ions, the ease of charge increases and hence, the bottom ion will reach the electrode first. (discussed further below)
| Positive Ions: | Negative Ions: |
| K+ | SO42- |
| Na+ | NO3– |
| Ca2+ | Cl– |
| Mg2+ | Br– |
| Zn2+ | I– |
| Fe2+ | OH– |
| Fe3+ | If the solution is concentrated, then OH– ion goes above the Cl– ion in the reactivity series. |
| Pb2+ | |
| H+ | |
| Cu2+ | |
| Ag+ | |
| Au+ |
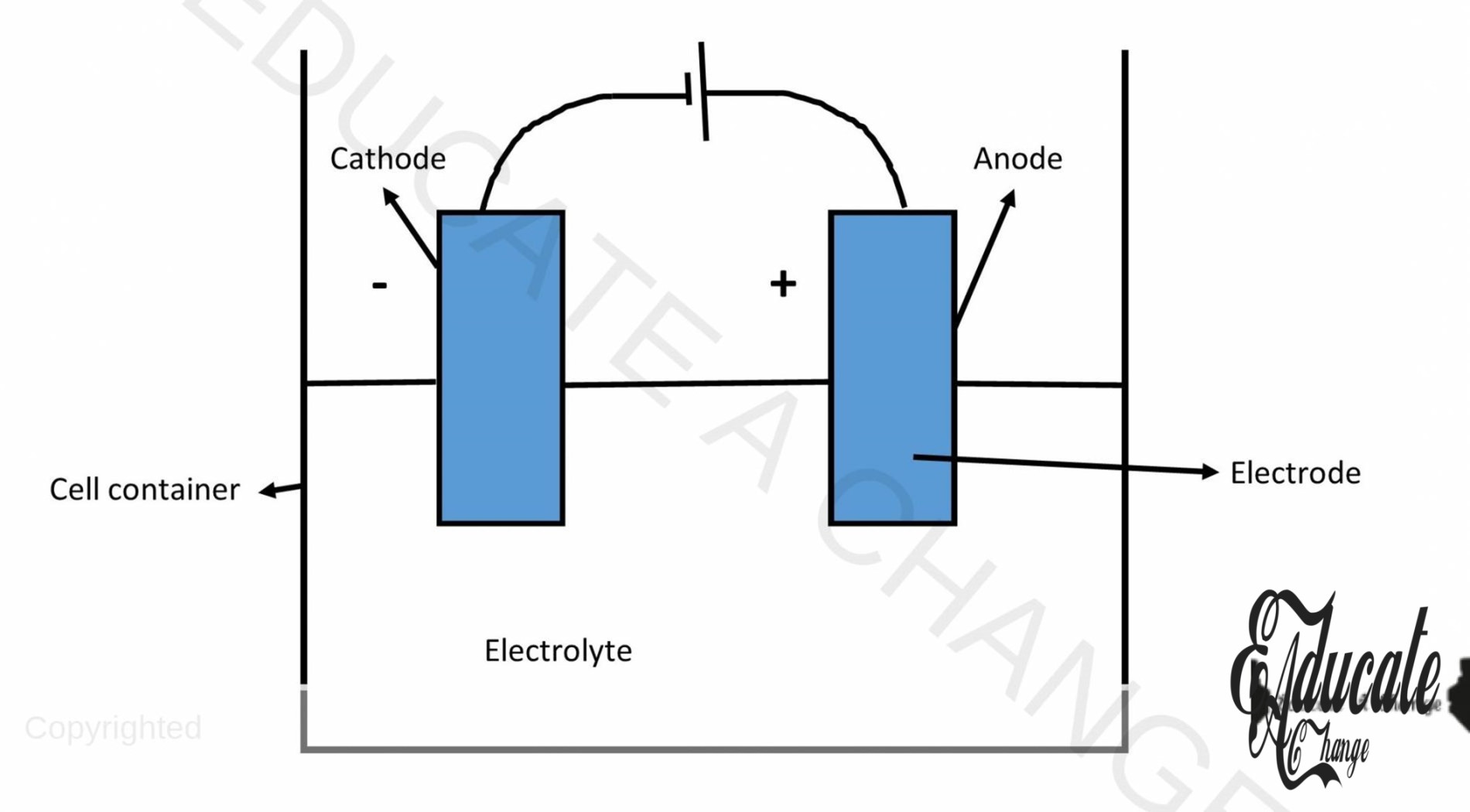
Anode and Cathode:
Anode:
It is the electrode/metal plate that is connected to the positive terminal of the cell. Anode is electron deficient and hence, the negative ions are attracted to the anode where they lose electron and become atoms.
Cathode:
Cathode is the electrode/metal plate that is connected to the negative terminal of the cell. Cathode is electron rich and hence, the positive ions are attracted to the cathode where they gain electron and become atoms.
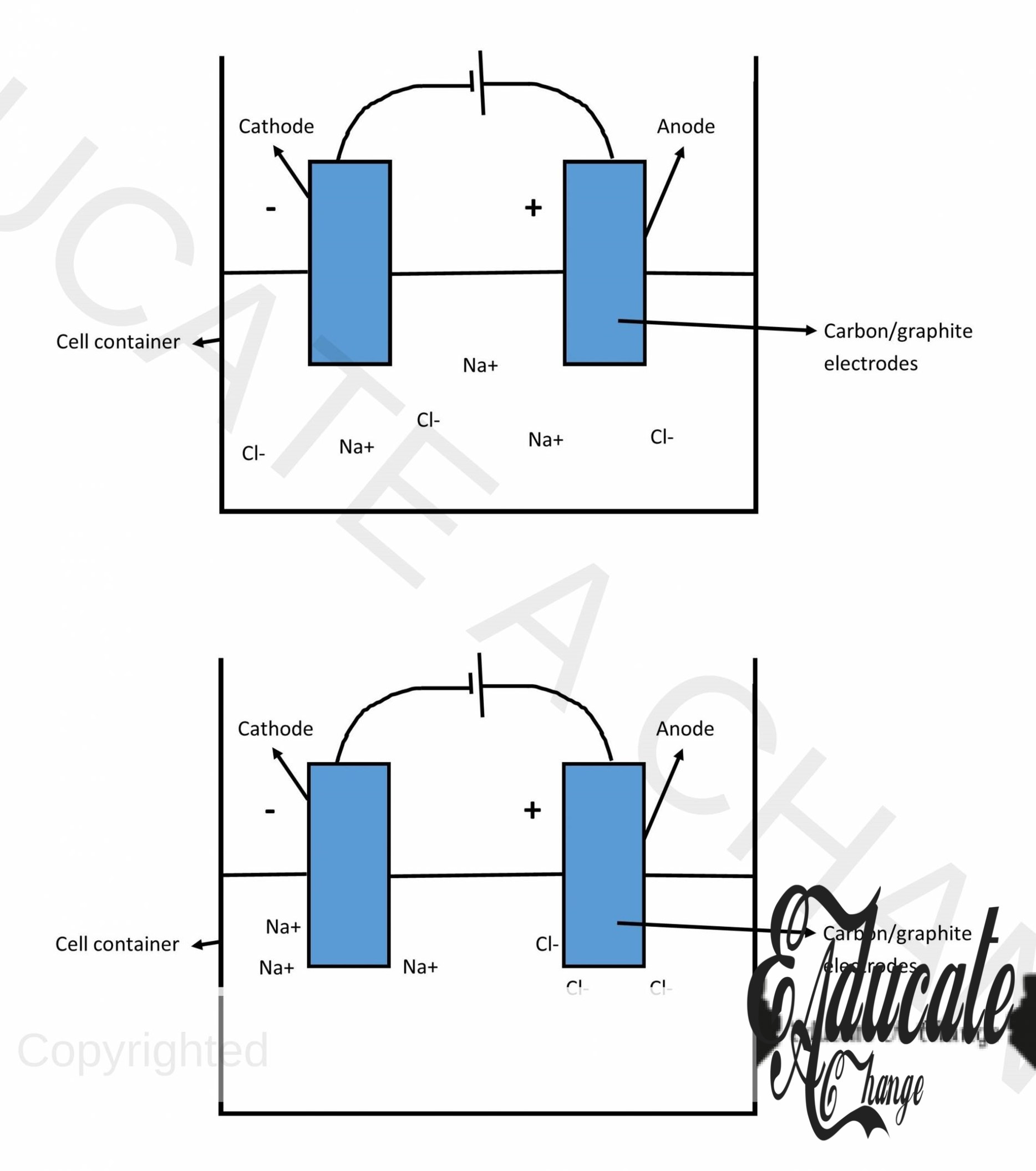
Electrolysis of molten Sodium Chloride (NaCl):
Expected Ions:
Na+, Cl–
Reaction at cathode:
Na+ + 1e– → Na (a greyish metal deposit at cathode)
Reaction at anode:
Cl– →Cl + e– Cl + Cl → Cl2 (a yellowish green gas will evolve)
Electrolysis of aqueous Sodium Chloride (NaCl aq.):
Expected Ions:
Na+, Cl–, H+, OH–
Reaction at cathode:
2H+ + 2e– → H2 (a colorless gas will evolve)
Reaction at anode:
Cl– →Cl + e– Cl + Cl → Cl2 (a yellowish green gas will evolve) Note that aqueous mean concentrated and hence, the OH ion will shift position in the reactivity series and go above chlorine which will result in chloride ion to reach anode instead of the OH– ion.
In solution:
The remaining ions in the solution will react to form a product. In this case, the Sodium and hydroxide ions form Sodium Hydroxide which will stay in aqueous form.
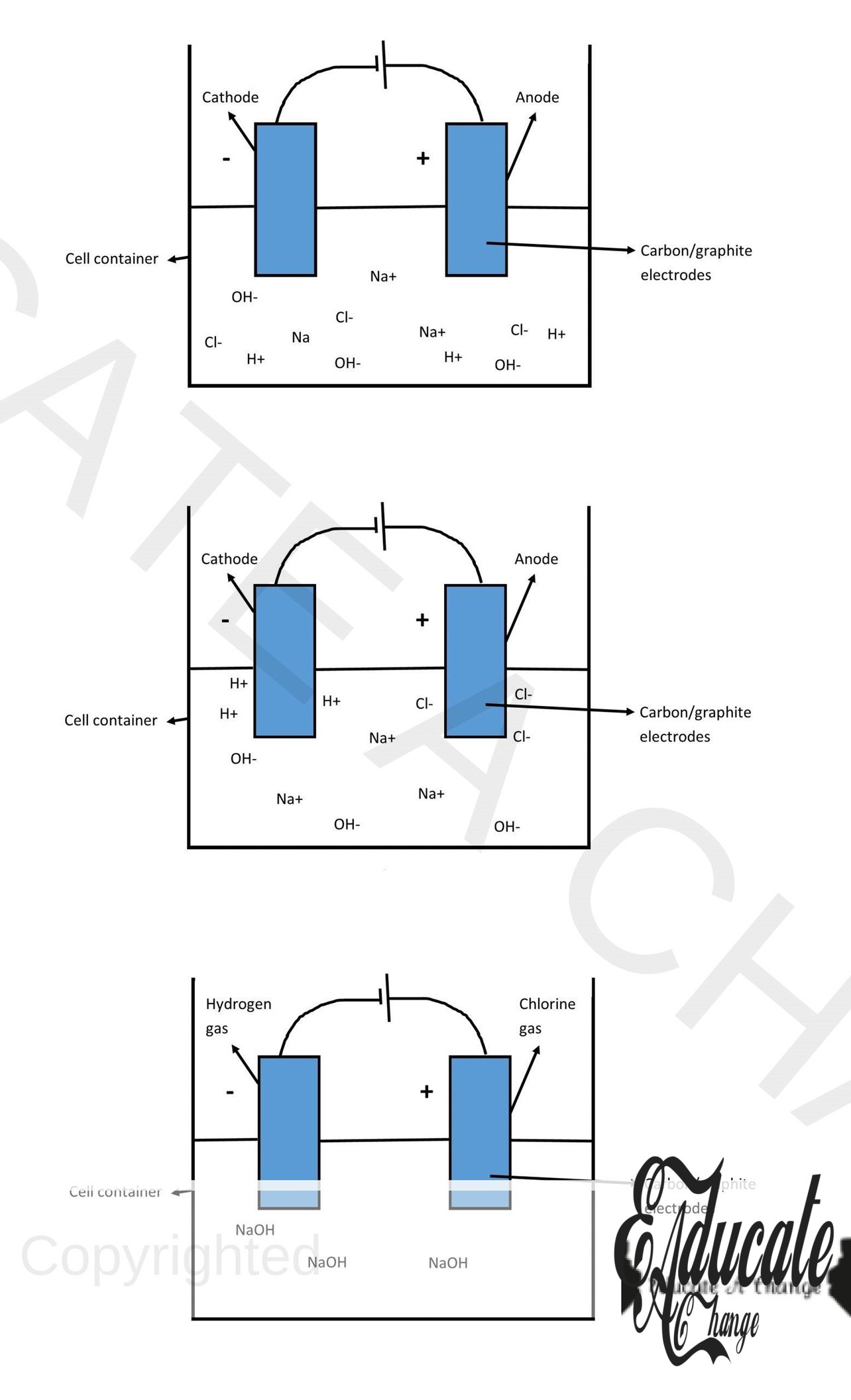
Electrolysis of dilute Sodium Chloride (NaCl aq.):
Expected Ions:
Na+, Cl–, H+, OH–
Reaction at cathode:
2H+ + 2e– → H2 (a colorless gas will evolve)
Reaction at anode:
4OH– →4OH + 4e– 4OH → 2H2O + O2 (oxygen evolves- relights burning splint)
In solution:
The remaining ions in the solution will be Sodium Chloride. As we can see, hydrogen and oxygen leaves and hence, the solution becomes more and more concentrated as time goes by.
Electrolysis of conc. Sulfuric Acid (H2SO4 aq.):
Expected Ions:
SO42-, H+, OH–
Reaction at cathode:
2H+ + 2e– → H2 (a colorless gas will evolve)
Reaction at anode:
4OH– →4OH + 4e– 4OH → 2H2O + O2 (oxygen evolves- relights burning splint)
In solution:
Since in this case, oxygen and hydrogen are being removed, the remaining solution will become more and more concentrated as electrolysis goes on.
Electrolysis of conc. Hydrochloric acid (HCl aq.):
Expected Ions:
Cl–, H+, OH–
Reaction at cathode:
2H+ + 2e– → H2 (a colorless gas will evolve)
Reaction at anode:
Cl– →Cl + e– Cl + Cl → Cl2
In solution:
Since in this case, chlorine and hydrogen are being removed, the remaining solution will contain Sodium hydroxide. Hence, the solution will increase in PH.
Factors affecting Electrolysis:
- Concentration,
- Ease of discharge,
- Type of electrode
Applications of Electrolysis:
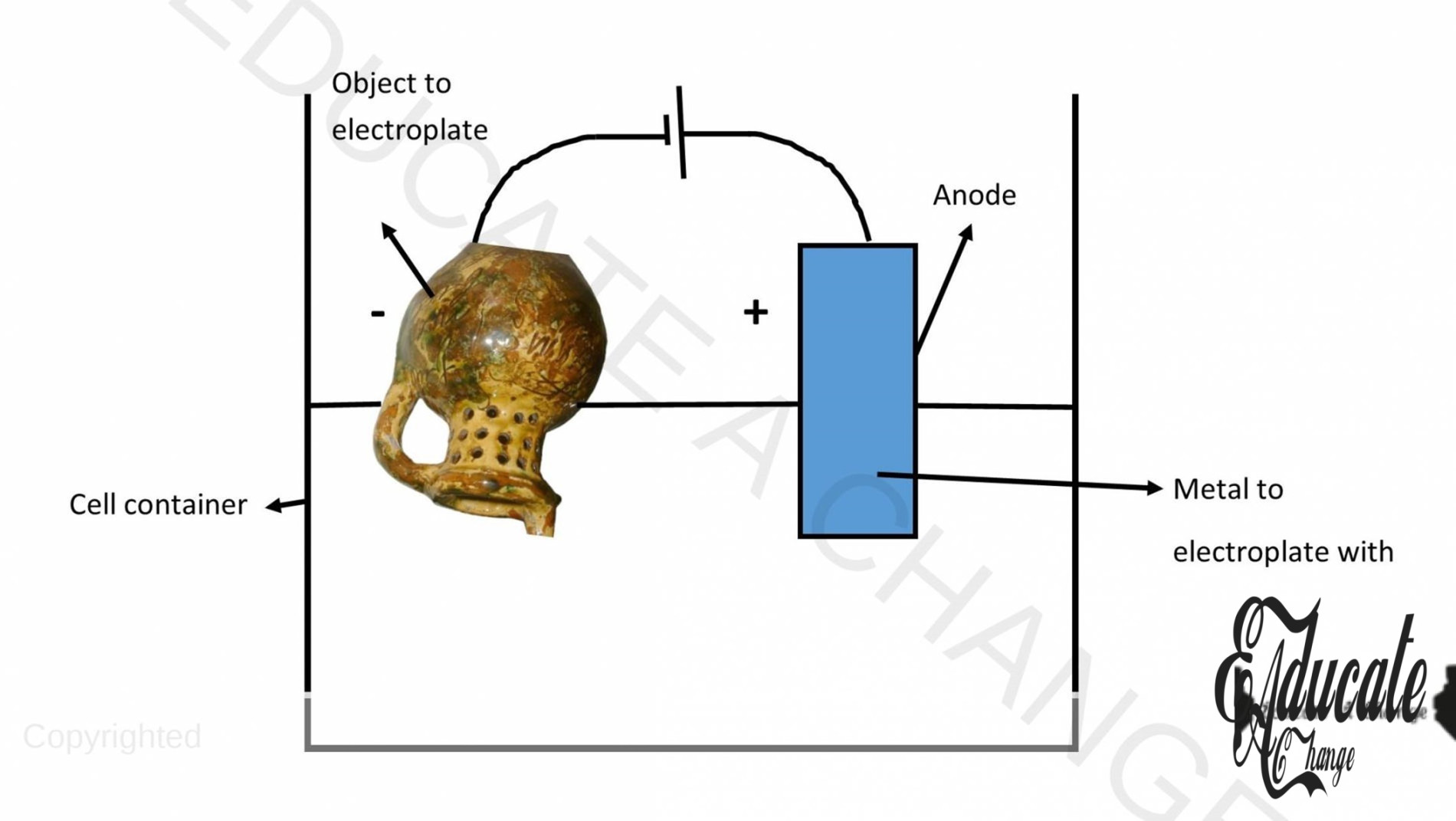
Electroplating:
When we want to cover one substance with a coat of another element, we attach the object as an electrode at the cathode, and the metal is placed in the solution where it has more ease to charge. Hence, the metal ions move to the cathode and get deposited at the object, creating a thin but firm coating. This is done to coat items with gold.
Batteries:
Our everyday use batteries are made by electrolysis. What happens is that we place two different elements with different ease of discharge as electrodes. The path from the cathode to the anode is the outer path of the battery. Inside the battery, the anode is where the negative ions lose electrons and these electrons move from the anode to the cathode which is electron rich. Positive ions move towards the cathode and gain electrons there. The movement of electrons from anode to cathode is the electric current.
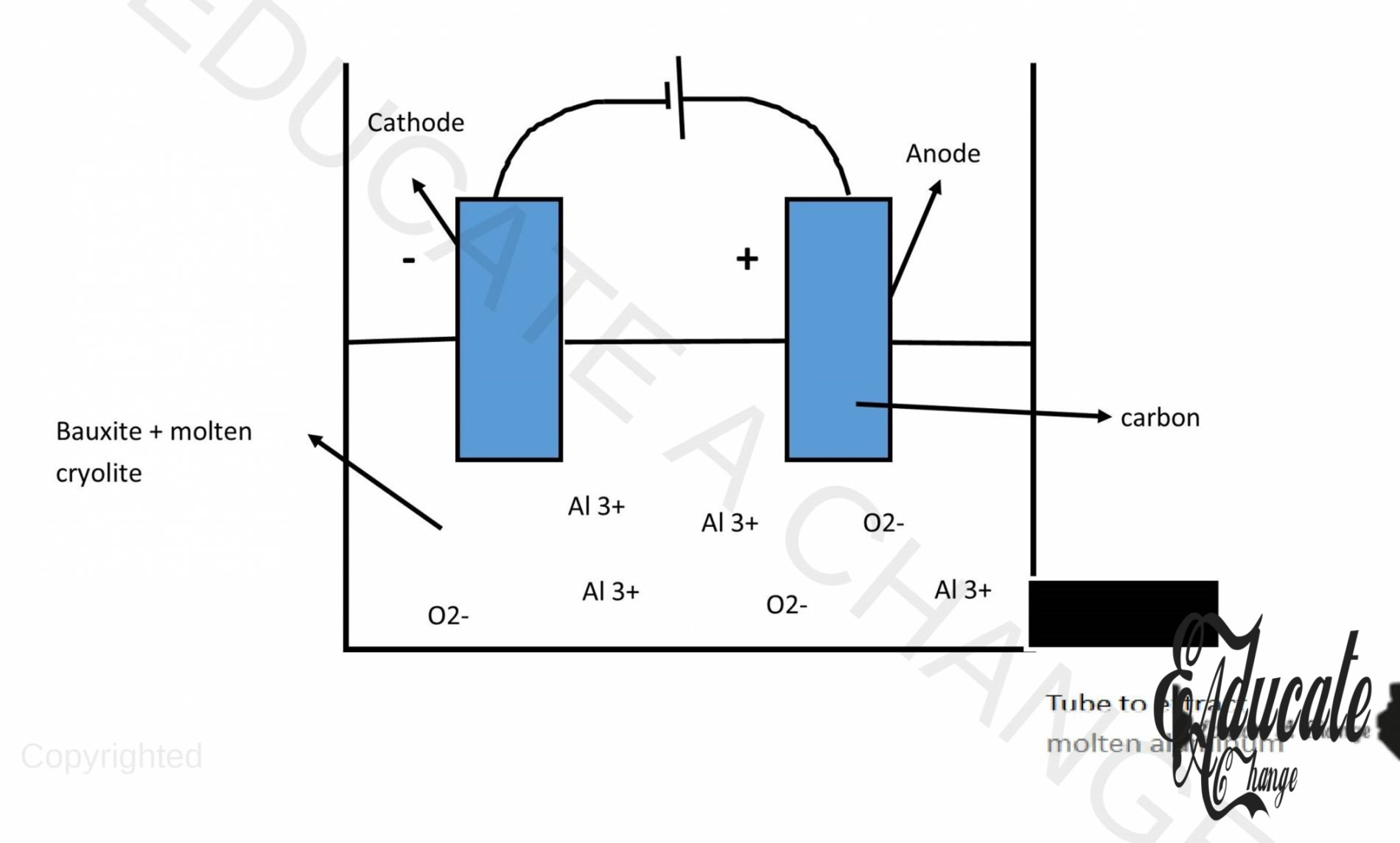
Production of Aluminum:
- Aluminum ore is called bauxite (Al2O3). We extract Aluminum from this ore. In order to extract it from its ore, we need to use electrolysis. However, since aluminum oxide has a high melting point, we do not melt t. Instead, we dissolve it in another aluminum compound called cryolite.
- At the cathode, aluminum ions receive electrons and turn into aluminum. Since aluminum has a charge of 3+, it takes in 3 electrons for each atom.:
- Al3++ 3e–→ Al
- On the anode, oxide ions are turned into oxygen gas:
- 2O2–→ O2+ 4e–
- However, since the electrode is made of carbon, the oxygen produced starts to react with the carbon and create carbon dioxide causing the electrode to corrode away. Hence, the electrode needs replacement after a while.
- The electrode replacement and the high electricity costs make aluminum expensive.