Organic Chemistry | O Level Chemistry 5070 & IGCSE Chemistry 0620 | Detailed Free Notes
Topics:
- Macromolecules
- Addition Polymerization
- Polyethene
- Uses of Poly Ethene
- Condensation Polymerization
- Nylon
- Terylene
- Uses
- Non-biodegradable plastics
- Natural Macromolecules
- Protein
- Fat
- Hydrolysis
Macromolecules:
- Macromolecules are very large structures that are built from small units called monomers.
- They are polymerized molecules as discussed earlier.
- Different types of macromolecules have different types of units or linkages
There are two types of Polymerizations as discussed below.
Addition Polymerization:
- Polymerization in which no small molecule is produced in addition to the polymer chain. I.e. nothing from the reactants leave the polymer chain.
- This happens when there are unsaturated monomers, i.e. they have double or triple bonds.
- We have seen one example of addition polymerization in the form of polyethene in alkenes
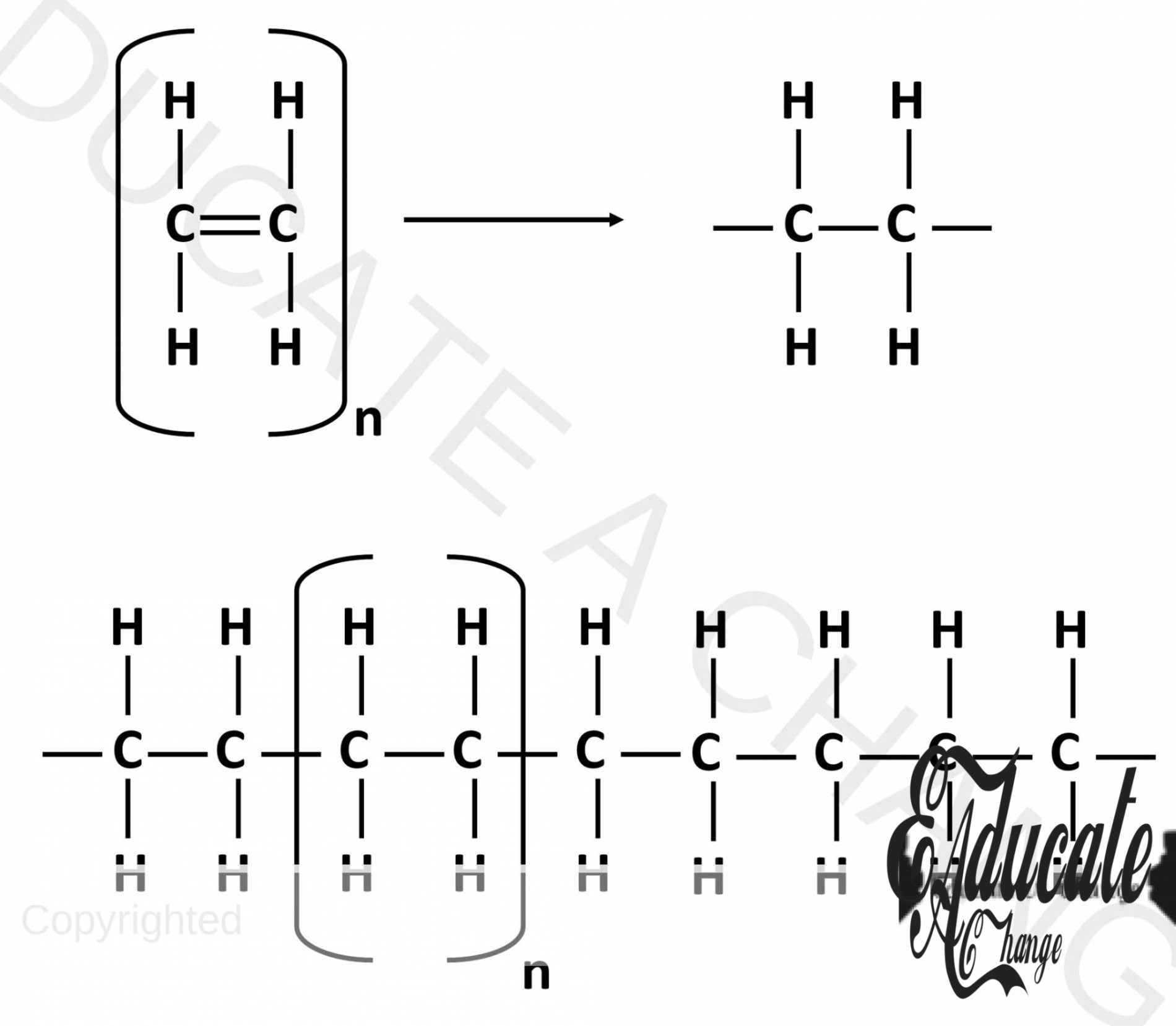
Polyethene:
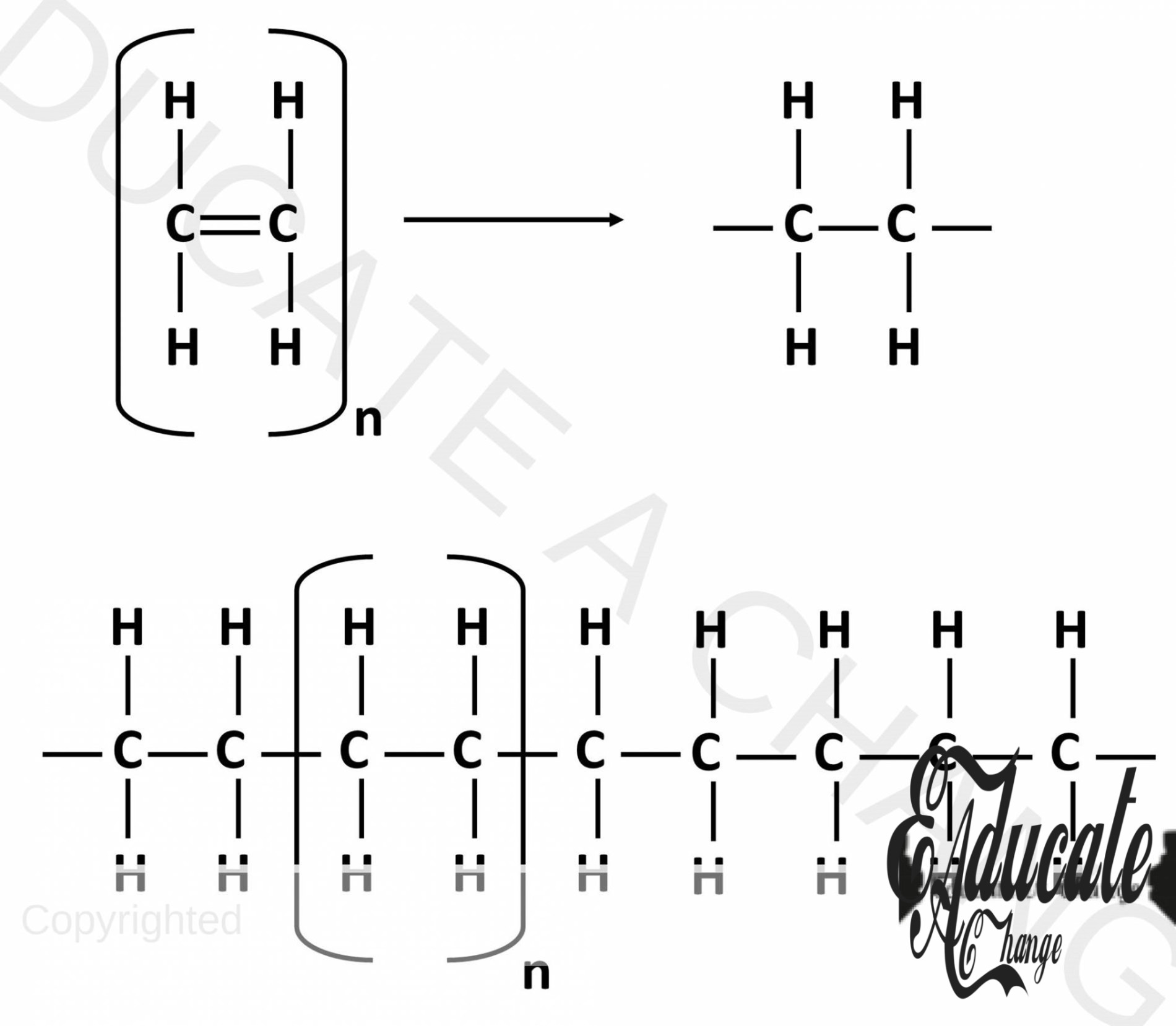
- Polyethene is formed when many ethene molecules join together.
- The double bond is broken, and the new available bonds join with other ethene molecules to form a large chain.
- This is shown in the diagram below. N represent a very large number. The unit in the brackets is one monomer. In this case, ethene.
Uses of Polyethene:
- We have heard the term polyethene bags. The most common use of polyethene is the making of plastic bags. Plastic is a polymer. Polyethene is one type of plastic.
- Polyethene is also used to make cling films.
Condensation Polymerization:
- Condensation polymerization is the type of polymerization where a small molecule is removed from the monomers to free the bond and create the chain.
- This small molecule can be anything such as water or HCl etc.
- There are three major Condensation polymers that we need to know: Polyamide, Nylon and Terylene.
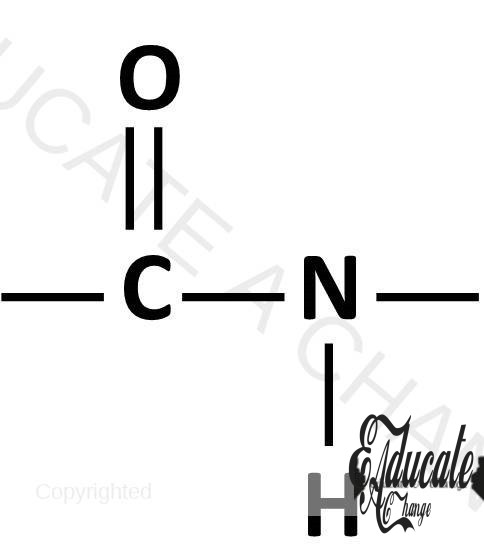
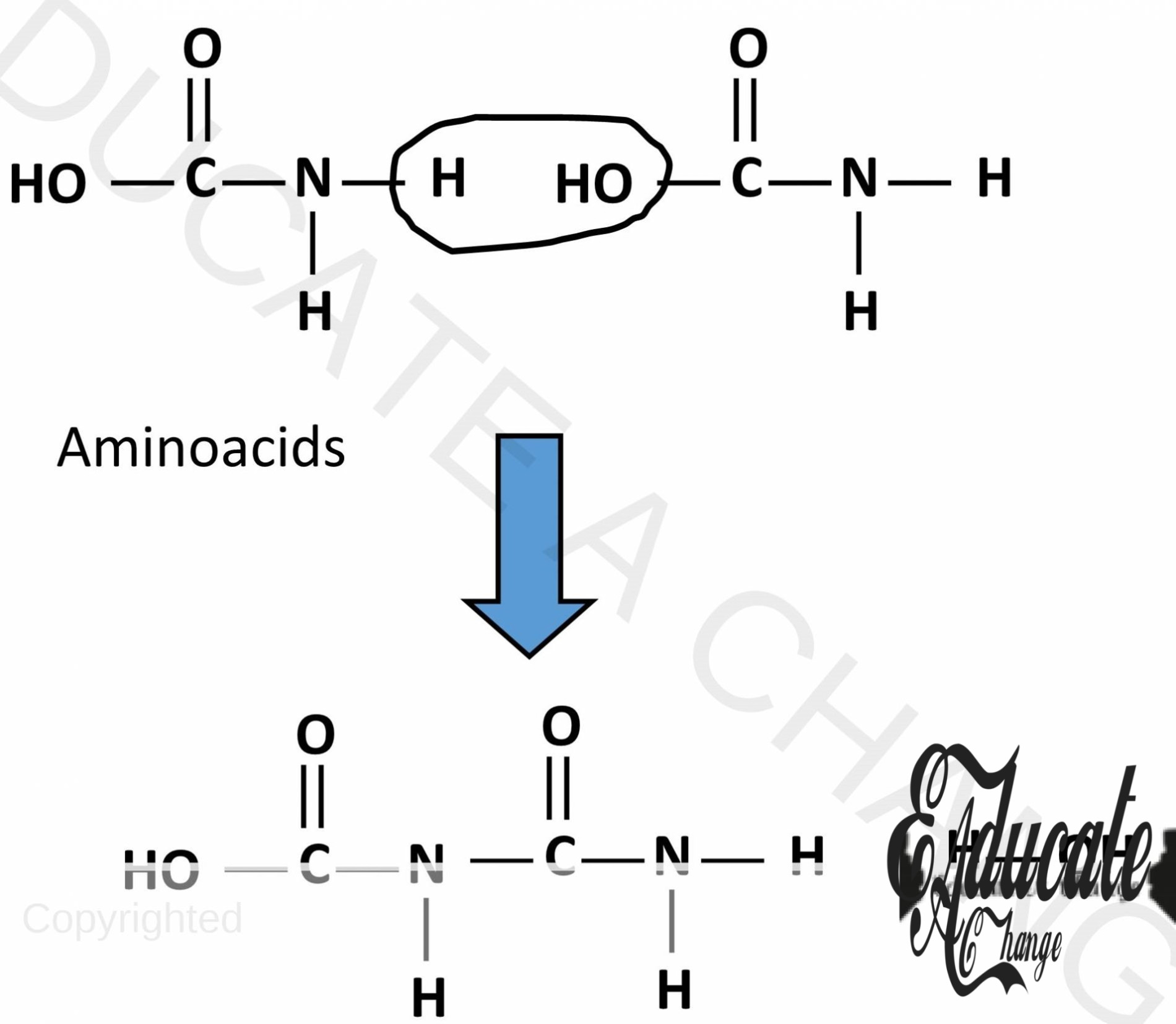
Nylon/ Polyamide:
Polyamide is a condensation polymer that contains the amide linkage. Amide linkage means -C=ONH- linkage.
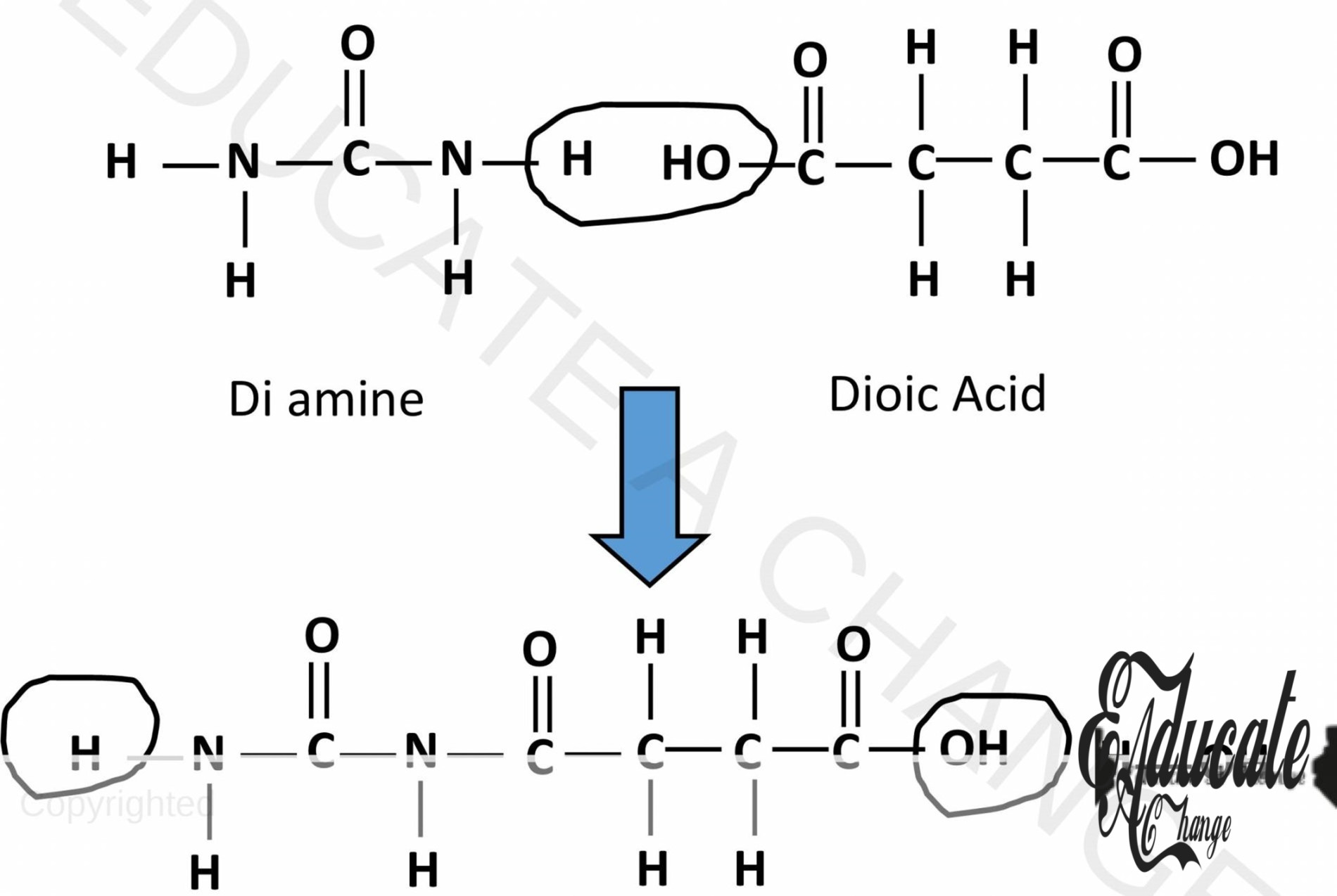
- Polyamide is formed when diamides and dicarboxylic acids react together.
- Water molecules are removed as carboxylic acid loses OH and amide loses H.
- They bond together.
- This can also happen on the other side creating a long chain.
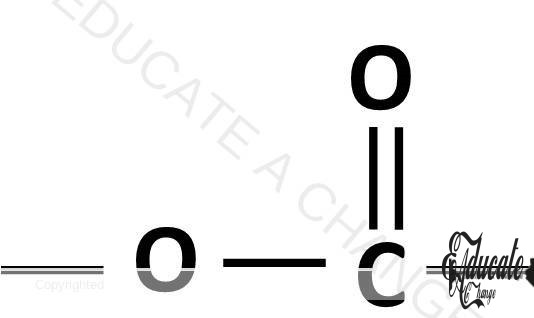
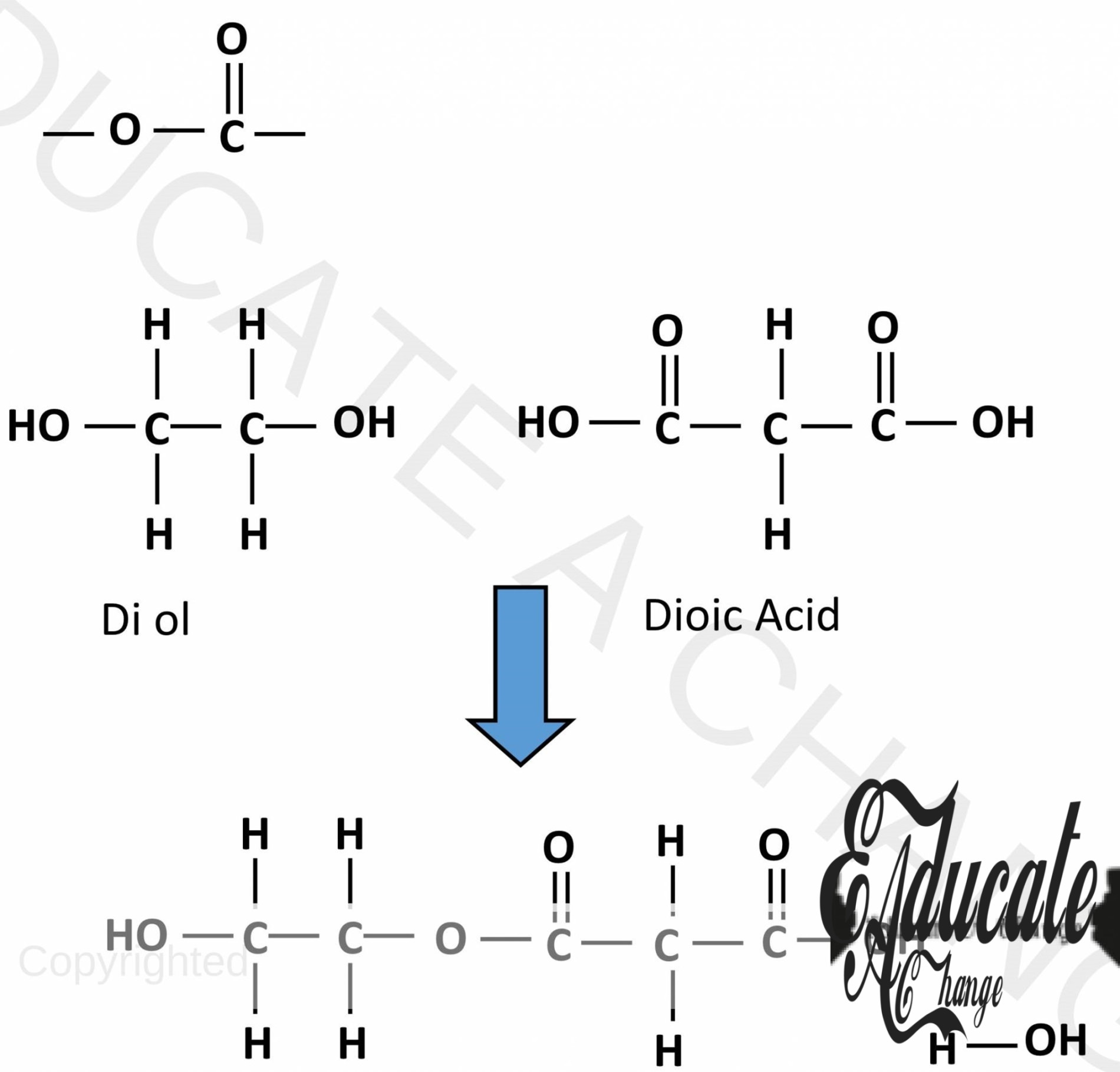
Terylene/ Polyester:
Polyester is a condensation polymer that contains the ester linkage. The ester linkage is O-C=O linkage.
- Polyester or terylene is formed when di-alcohols or diols and dicarboxylic acids react together.
- Water molecule is removed, and they bond together to form esters.
- This can also happen on the other side creating a long chain polymer.
Uses of Polyamide and Polyester:
There are various uses of Terylene and Nylon. These include but are not limited to clothing, materials, fishing lines, parachutes and sleeping bags.
Non-biodegradable Plastics:
Plastics that are non-biodegradable cause serious pollution as they trap in ground or water and stop the passage and flow of water. They take very long to decompose. Hence, their use is being reduced and banned. Recycling plastics is another option that is now being used.
Natural Macromolecules:
Polyethene, polyamide and polyesters are created in industries for specific purposes. However, there are several natural macromolecules. These include Protein, carbohydrates and fats.
Proteins:
Proteins have the same amide linkage as in nylon. However, their monomers are different to those of nylon. IN proteins, the amide linkage is called the peptide bond. Proteins are formed by the polymerization of amino acids that contain the amine structure on one side and carboxylic structure on the other.
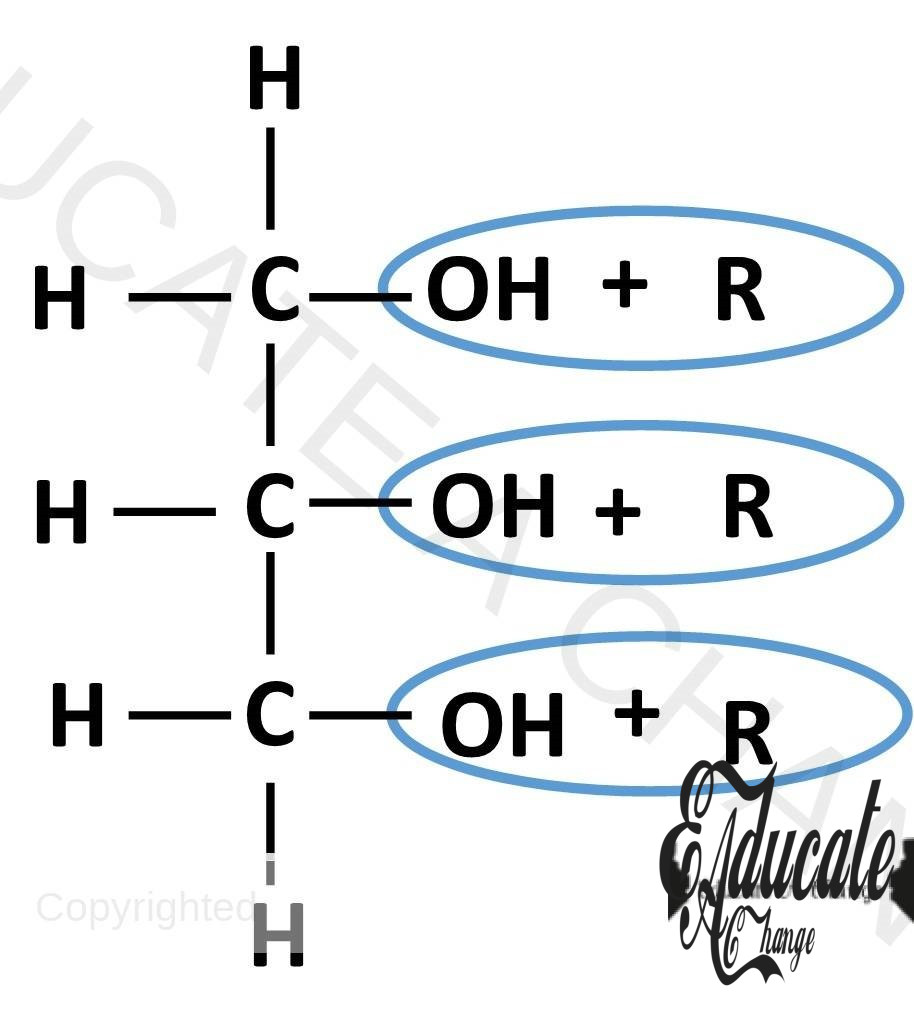
Fats:
- Fats have the same ester linkage. However, the monomers for fats are difference to those of polyesters.
- The fats have three fatty acids combined with glycerol molecule to form long chains.
- Since we add three fatty acids with glycerol, fats are called triglycerides.
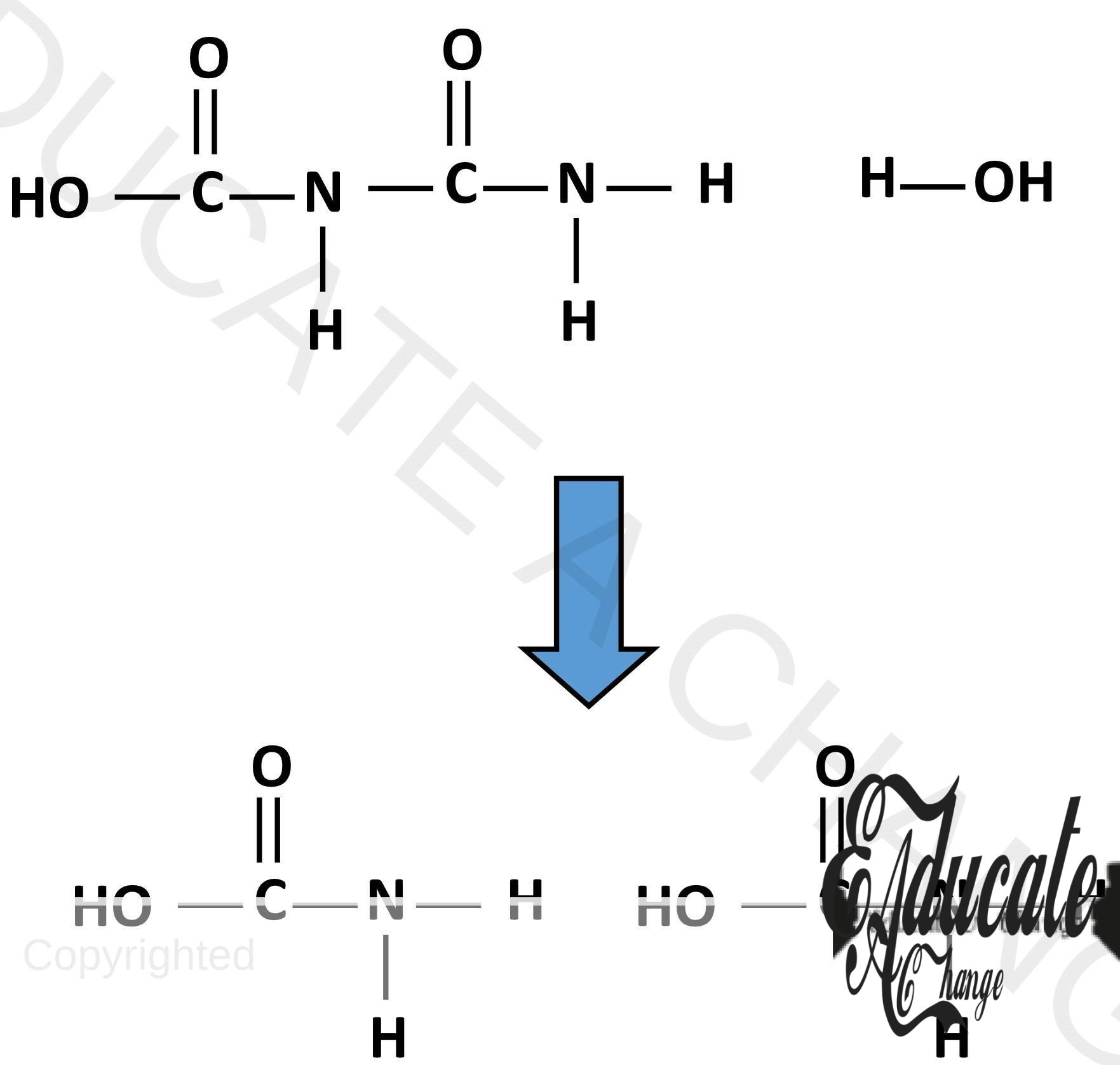
Hydrolysis:
Hydrolysis is the breaking down of proteins back into amino acids in the presence of water. The bonds that were formed are replaced with H and OH and amino acids are formed again. Complex carbohydrates are also hydrolyzed for example, starch into sugar.
Topics:
- Alcohol
- Alcohol
- Structures of Alcohol
- Drawing the structure of alcohol
- General formula
- Formation of Alcohols
- Combustion
- Oxidation of Alcohols
- Uses of Alcohols
- Carboxylic Acid
- Structure of Carboxylic acids
- Drawing Carboxylic acids
- Formation of Carboxylic Acids
- Reaction of Carboxylic Acids
- Esterification
Alcohol
Alcohol:
- Alcohols are a homologous series of compounds that contain OH functional group (called hydroxyl group).
- Alcohol naming convention is similar to Alkanes and Alkenes: Methanol, Ethanol, Propanol, Butanol and so on.
- Alcohols are soluble in water.
- Keep in mind that alcohols are not alkalis even though they have OH in them.
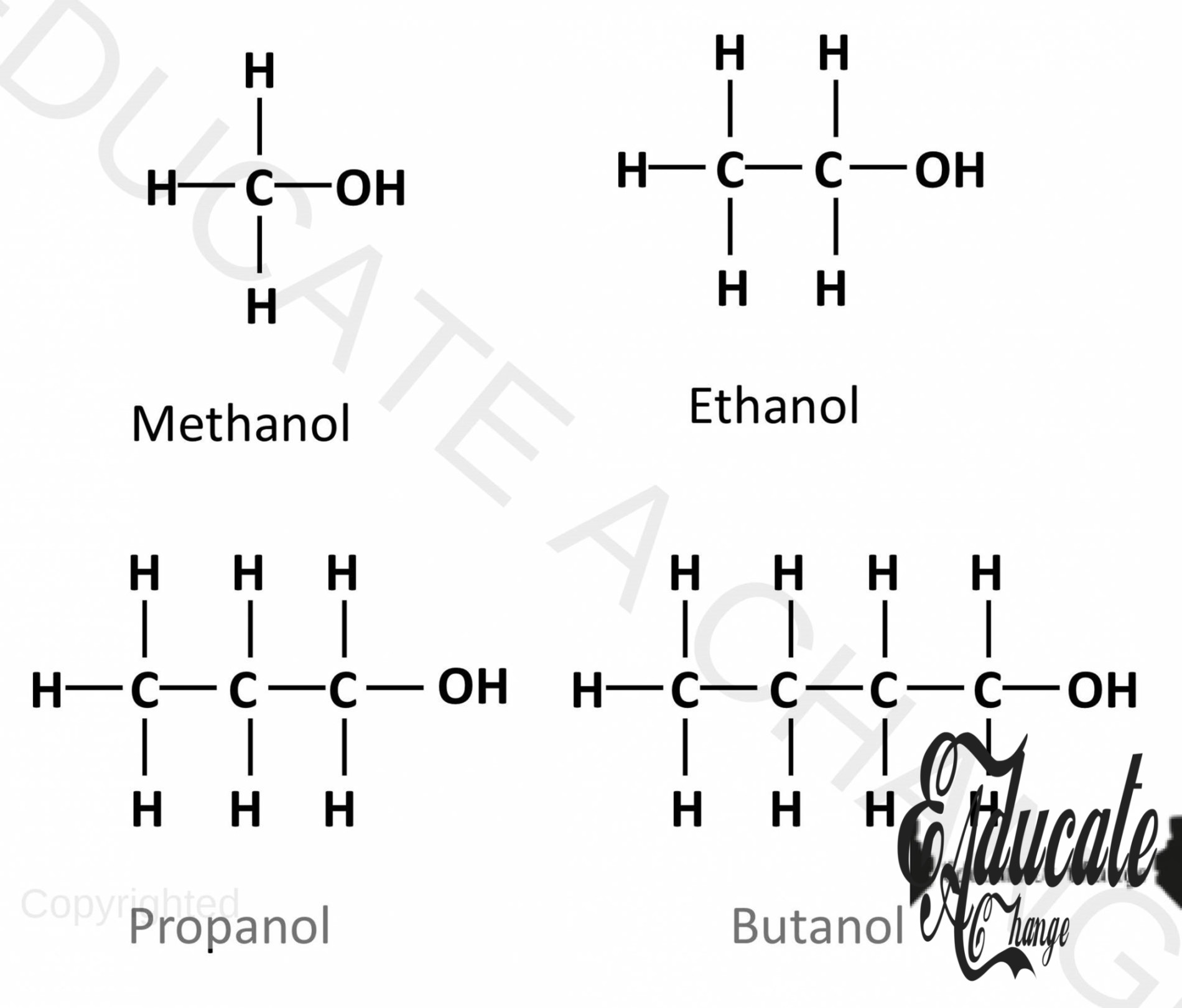
Structures of Alcohols:
The following diagram shows the structures of the first four alcohols.
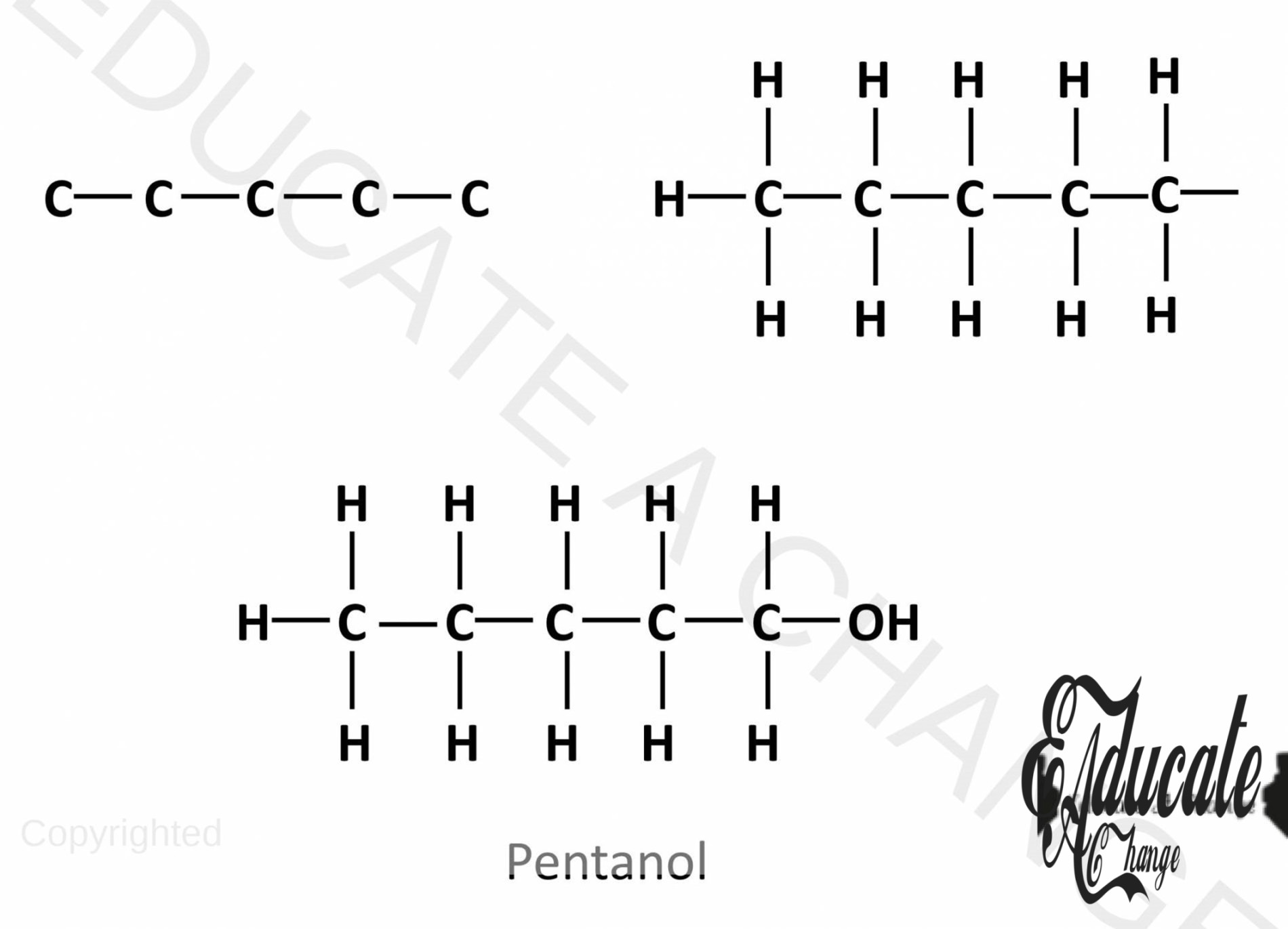
Drawing the structure of Alcohols:
- Let’s say we are to draw the structure of pentanol, 5 carbon alcohol.
- We draw a straight chain of carbons. The corner carbon will have 3 hydrogens and all the other carbons will have 2 hydrogens.
- The last carbon will have OH attached to it as well.
General Formula:
Alcohols have the general formula: CnH2n+1OH SO for example, we have 4 carbons i.e. butanol: C4H2(4) +1OH = C4H9OH Which can be verified from the above diagram.
Formation of Alcohols:
As discussed earlier in previous lecture, alcohols are formed by the addition of steam to alkenes with the presence of a catalyst. Ethanol is also formed by the fermentation of Glucose. This is carried out my micro-organisms.
- We use a moderate 37 degrees temperature since too high temperature will kill yeast.
- Make sure that oxygen does not react with the alcohol and turn it into carboxylic acid.
- We keep checking the alcohol formation using the limewater test of carbon dioxide.
Combustion:
Alcohols burn in oxygen to produce carbon dioxide and water. E.g. C4H9OH + 6.5O2 → 4CO2 + 5H2O
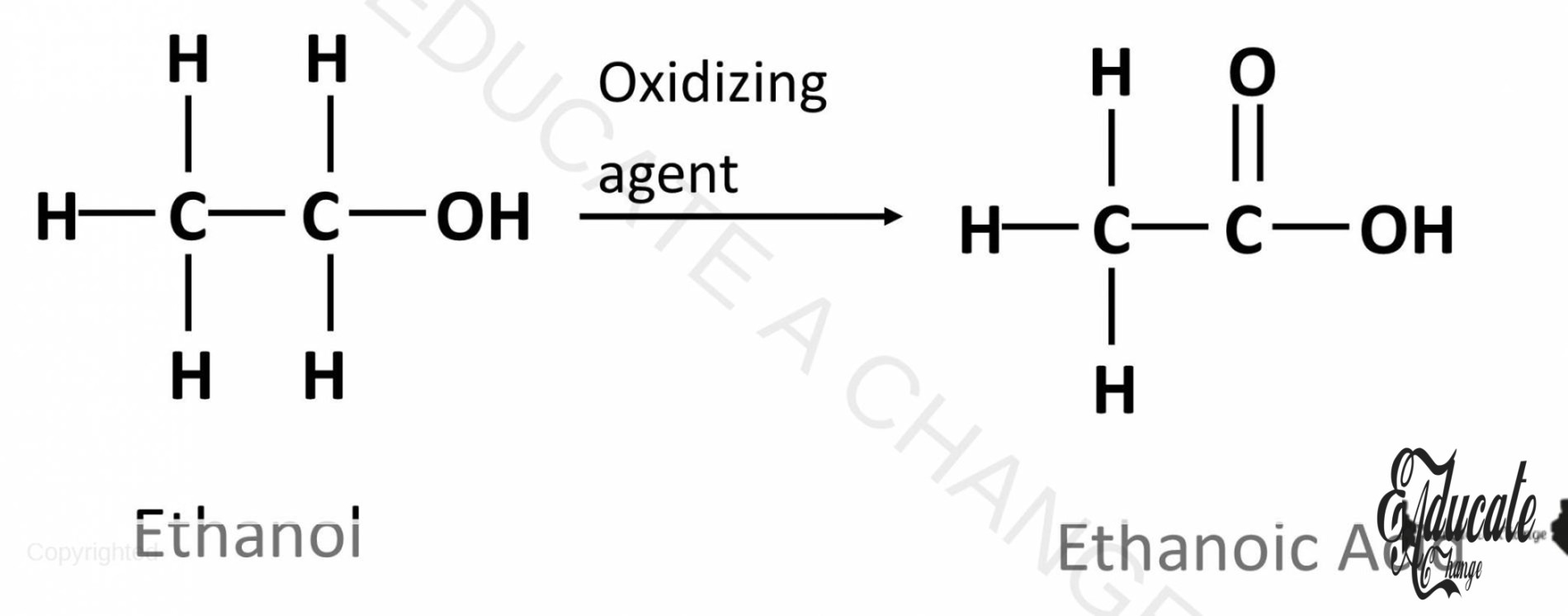
Oxidation of Alcohols:
Alcohols are oxidized into carboxylic acids. This happens in the presence of acidified Potassium Dichromate (H2SO4 K2Cr2O7) which acts as the oxidizing agent. E.g. Ethanol can be converted to Ethanoic Acid.
Uses of Alcohol:
- It is used as a solvent for, for example, detergents and perfumes
- Alcohol can also be used as a fuel since it burns.
- It is used as antiseptics.
- Drinks and beverages.
Carboxylic Acids:
- Carboxylic Acids are a homologous series that contain the COOH functional group.
- They are weak acids that partially ionize.
- They are soluble in water.
- Carboxylic Acids have to groups, the hydroxyl group (OH) and the carbonyl group (C=O).
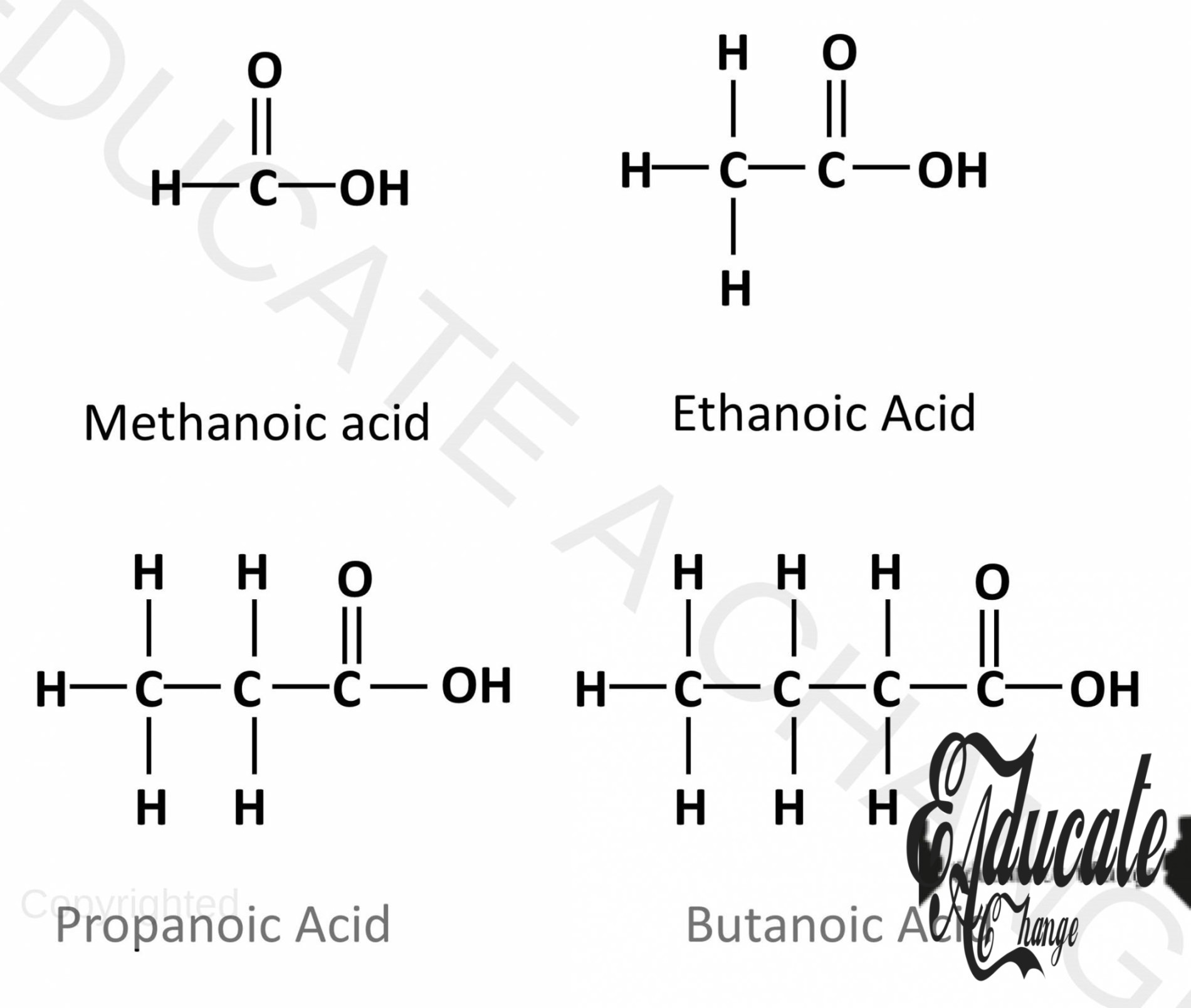
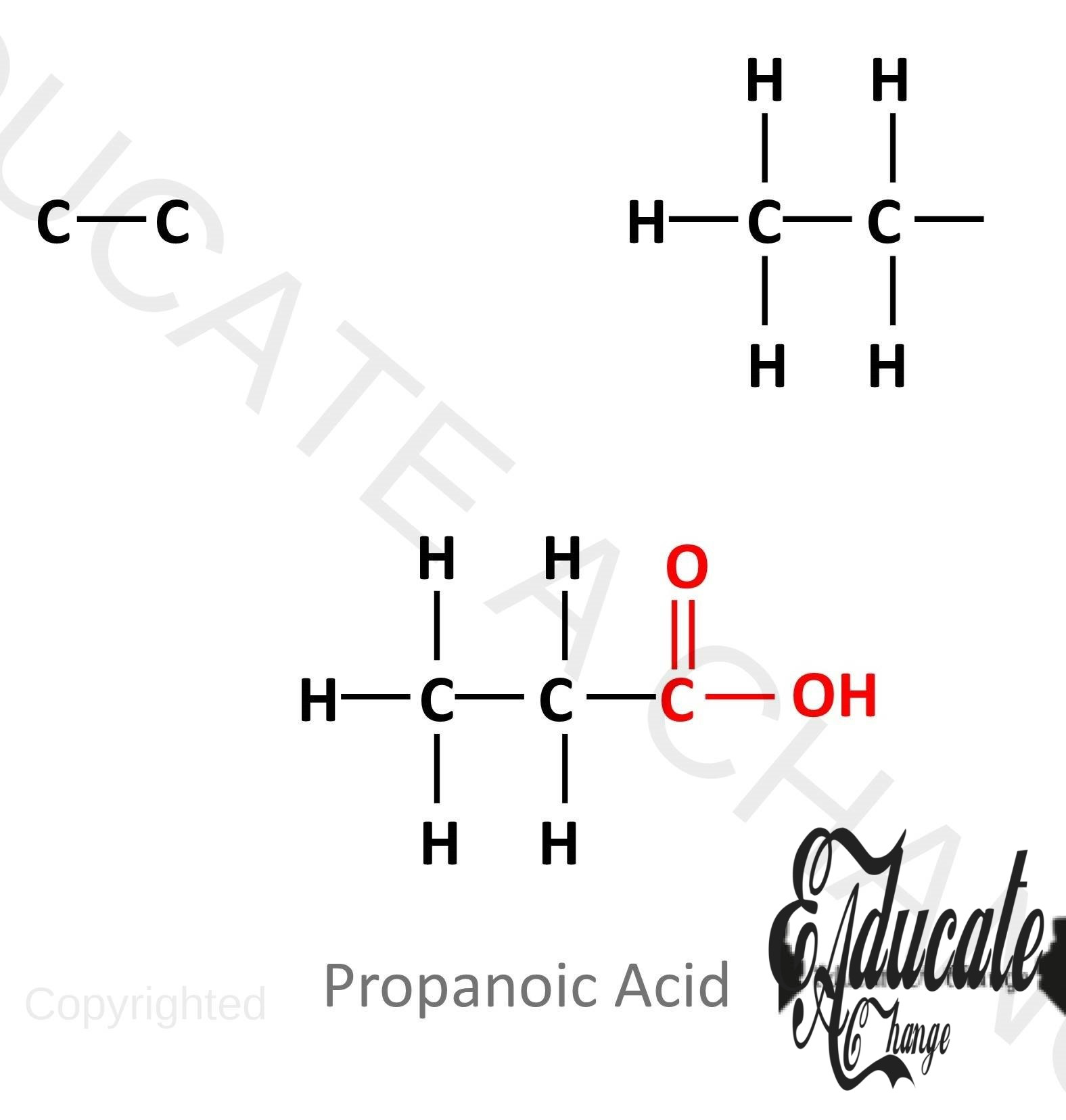
Structures of Carboxylic Acids:
The following diagram shows the structures of the first four Carboxylic Acids.
General Formula of Carboxylic Acids:
Carboxylic Acids have the general formula: CnH2n+1COOH SO for example, we have 4 carbons i.e. butanoic acid, we will count the 3 carbons in the first part as one is in the COOH Part: C3H2 (3) +1COOH = C3H7COOH Which can be verified from the above diagram.
Drawing the structure of Carboxylic Acids:
- Let’s say we are to draw the structure of propanoic acid, 3 carbon carboxylic acid.
- We draw a straight chain of carbons one less than the number needed. Here we draw 2. The corner carbon will have 3 hydrogens and all the other carbons will have 2 hydrogens.
- The last carbon will have COOH attached to it as well.
Formation of Carboxylic Acids:
As discussed above, carboxylic acids are formed by the oxidation of alcohols using acidified catalysts that act as oxidizing agents. This can be Potassium Dichromate or Potassium Permanganate. Also, while fermentation, if oxygen is added, acids are produced, ethanol.
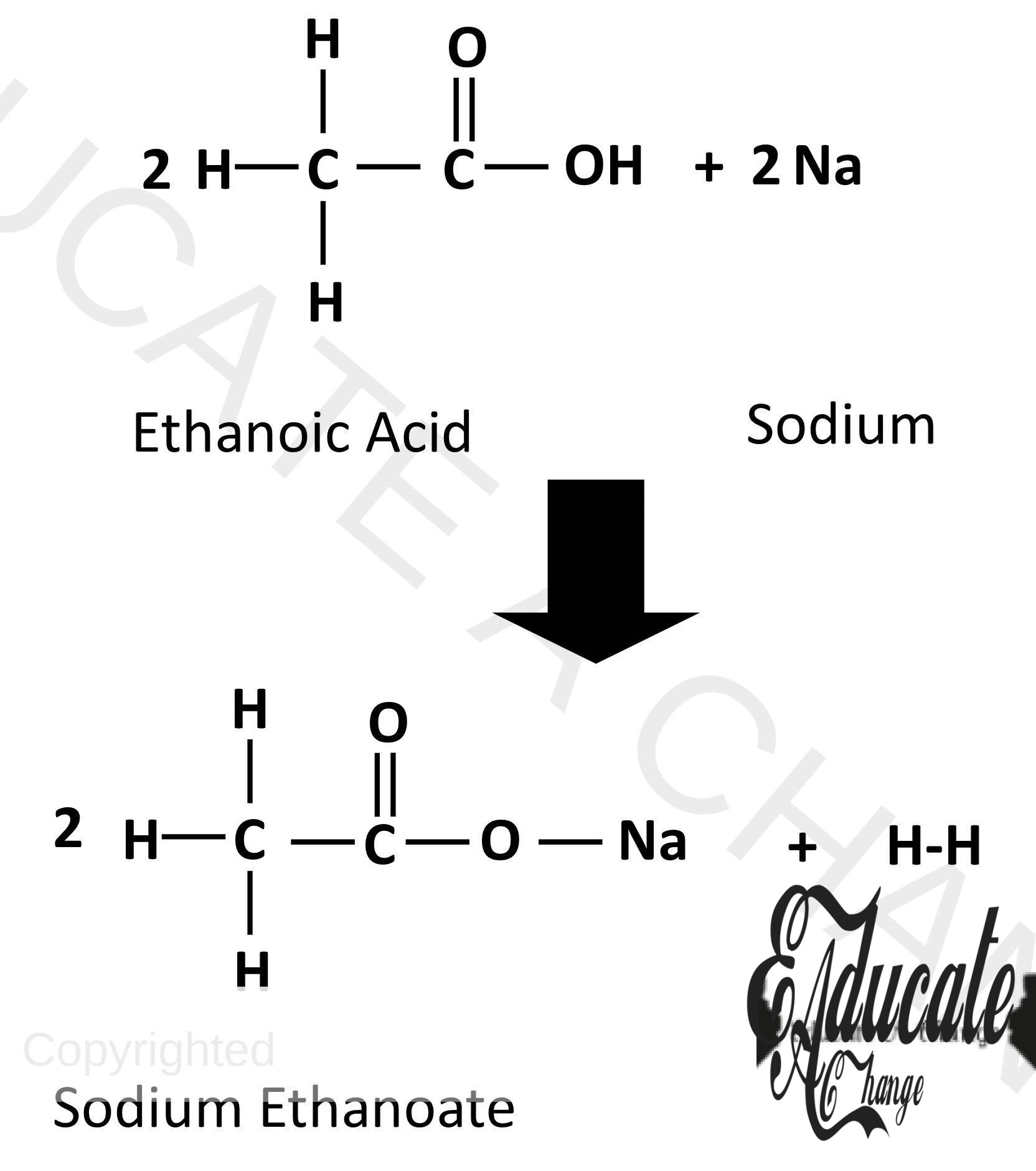
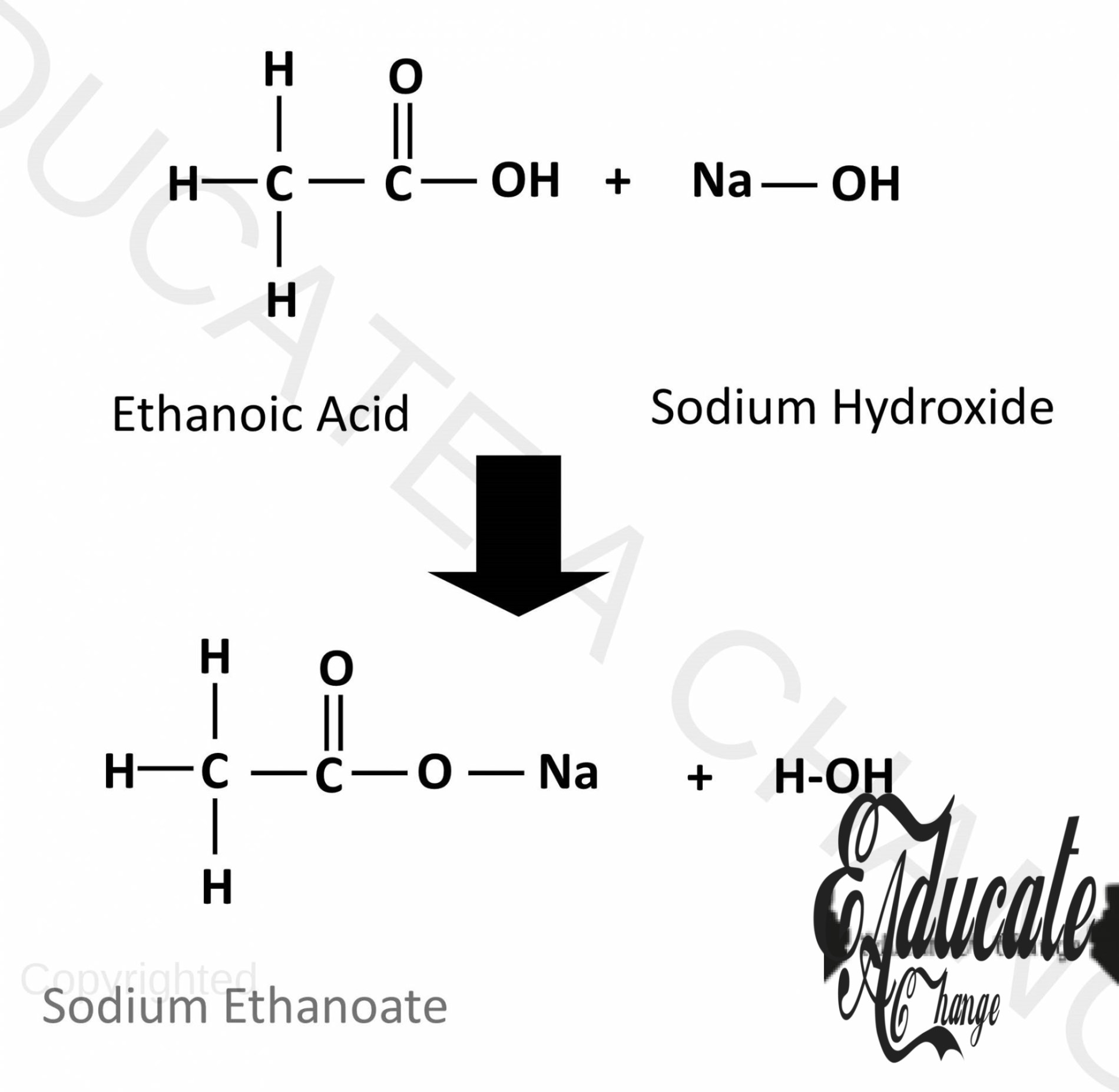
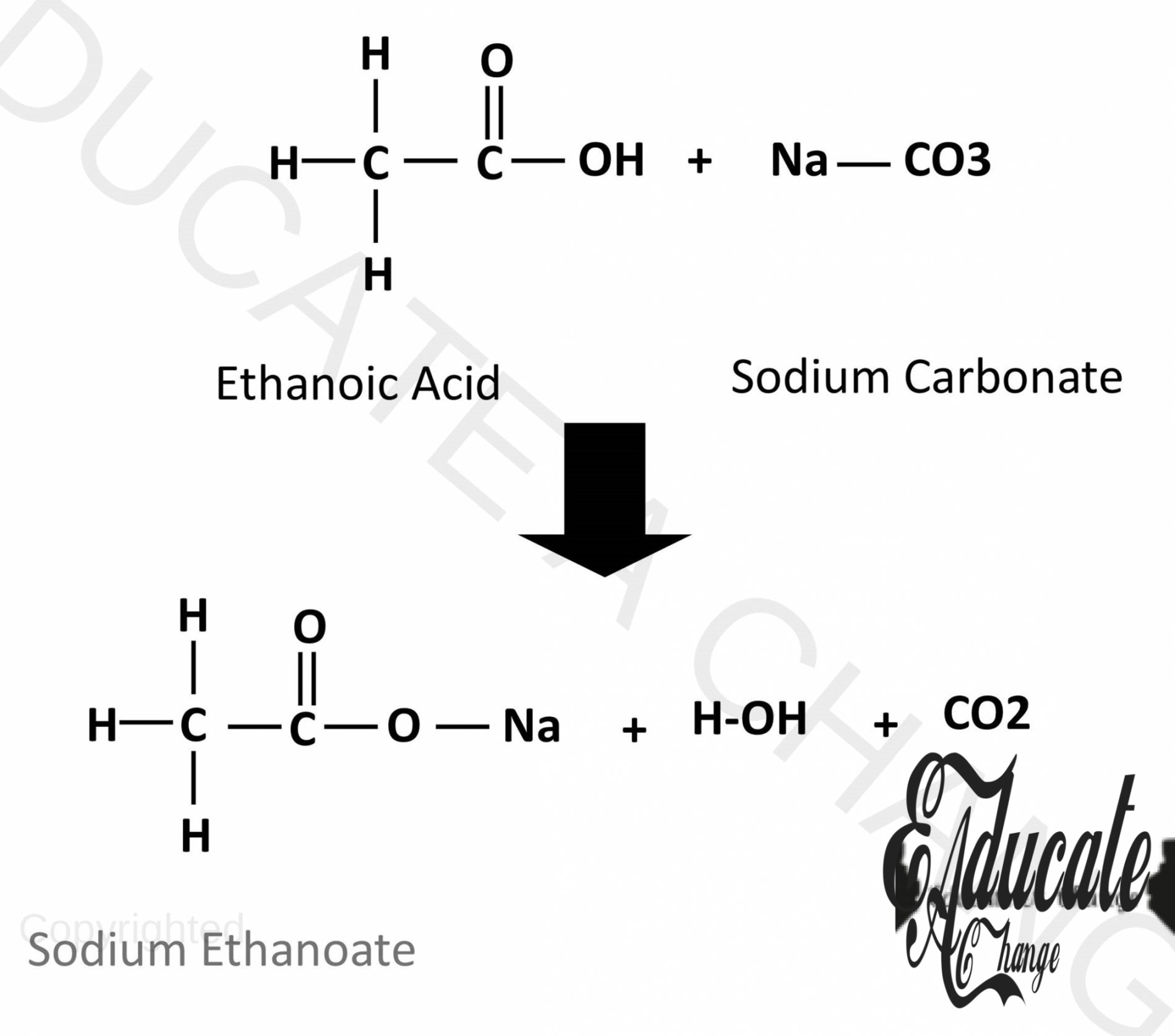
Reactions of Carboxylic Acids:
Since they are weak acids, they react with some carbonates, bases and metals in the similar way the other acids react.
Metals:
They donate the H (as acids do) and form salts and hydrogen with metals:
Bases:
They neutralize and produce salt and water with bases:
Carbonates:
They product salt, water and carbon dioxide with carbonates.
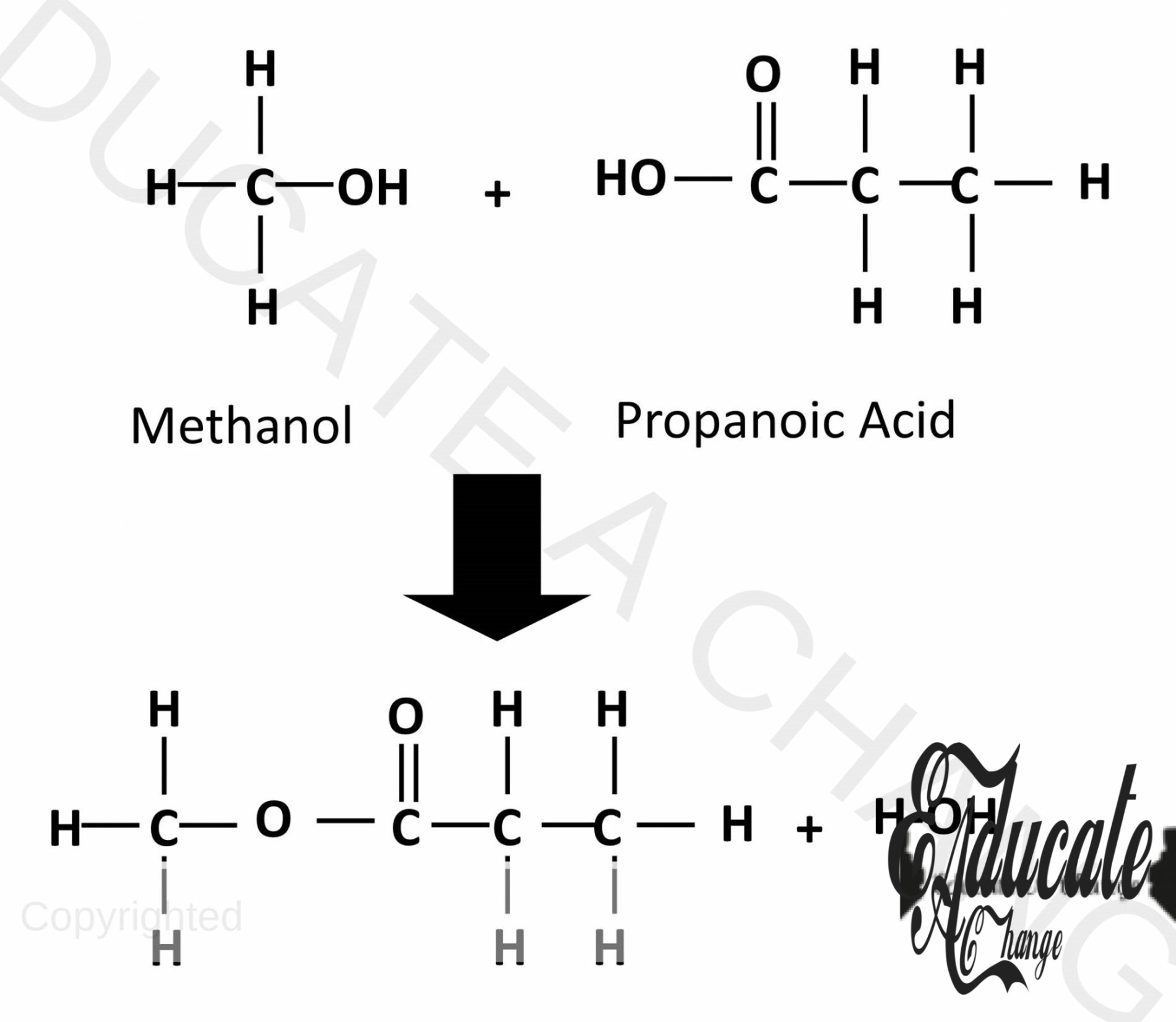
Esterification:
- Carboxylic acids and alcohols react together to form sweet-smelling organic compounds called esters.
- Esters are used in artificial flavoring and perfumes as well.
- Acids donate H and alcohols donate OH and hence, water is also formed together with the esters.
- They are called alkyl carboxylate e.g. when methanol and propanoic acid form an ester, it will be called methyl propanoate.
- CH3CH2COOH + CH3OH → CH3CH2COOCH3
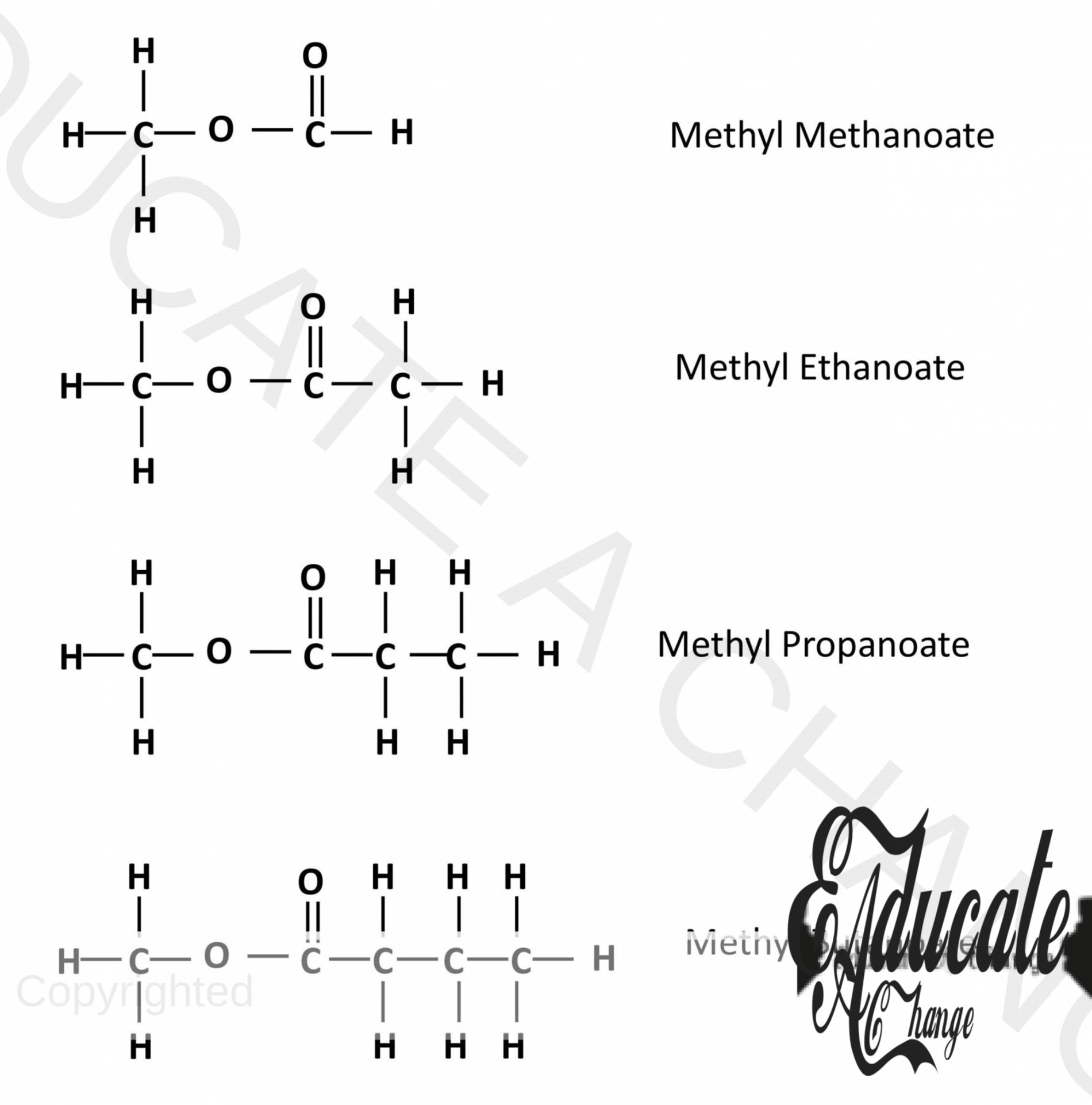
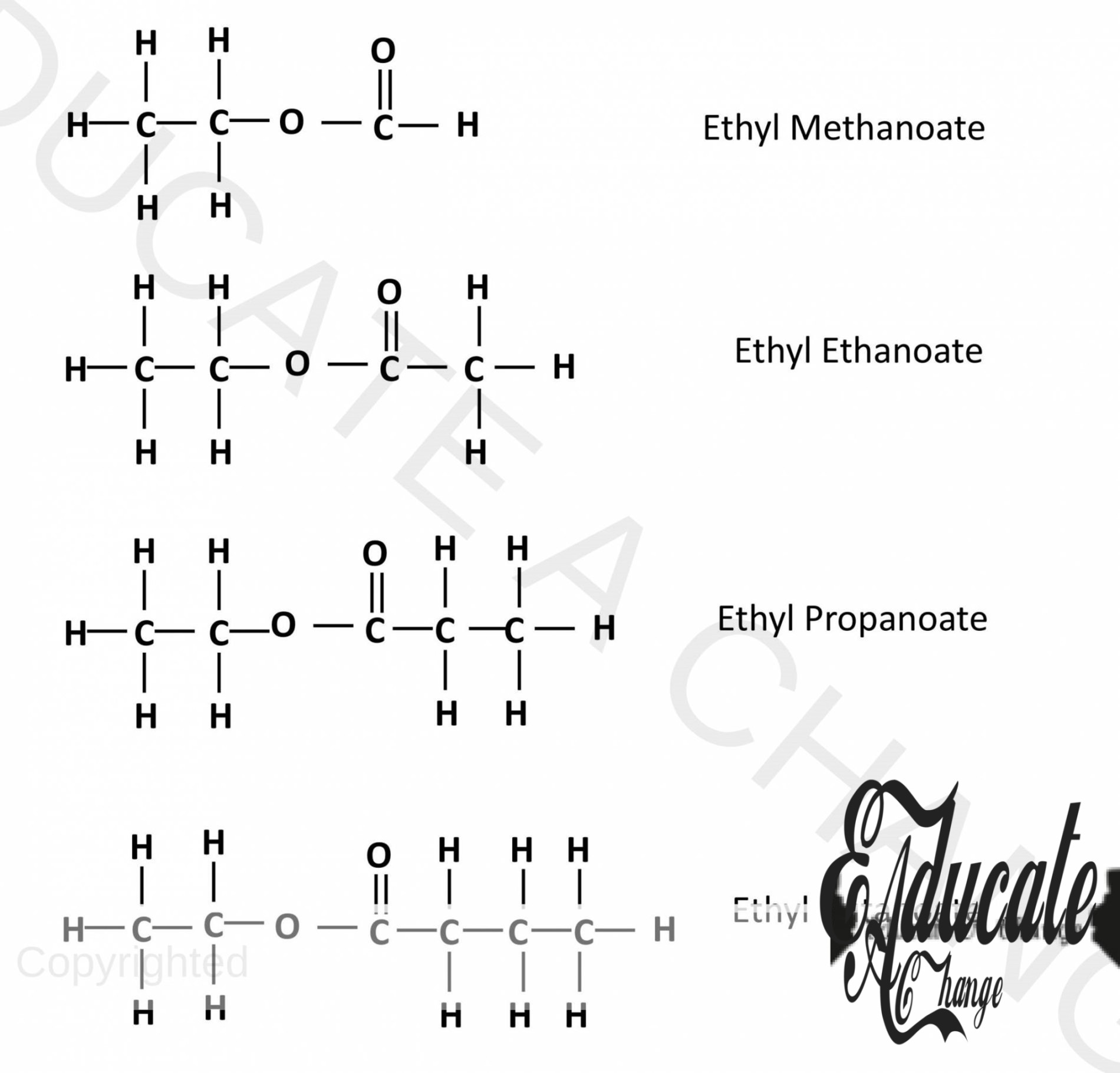
Structures of esters:
The following diagram shows the structures of esters of the first 4 carbon compounds:
Esters with Methanol and acids:
Esters with Ethanol and acids:
Similarly, we can make with propanol and butanol with all acids.
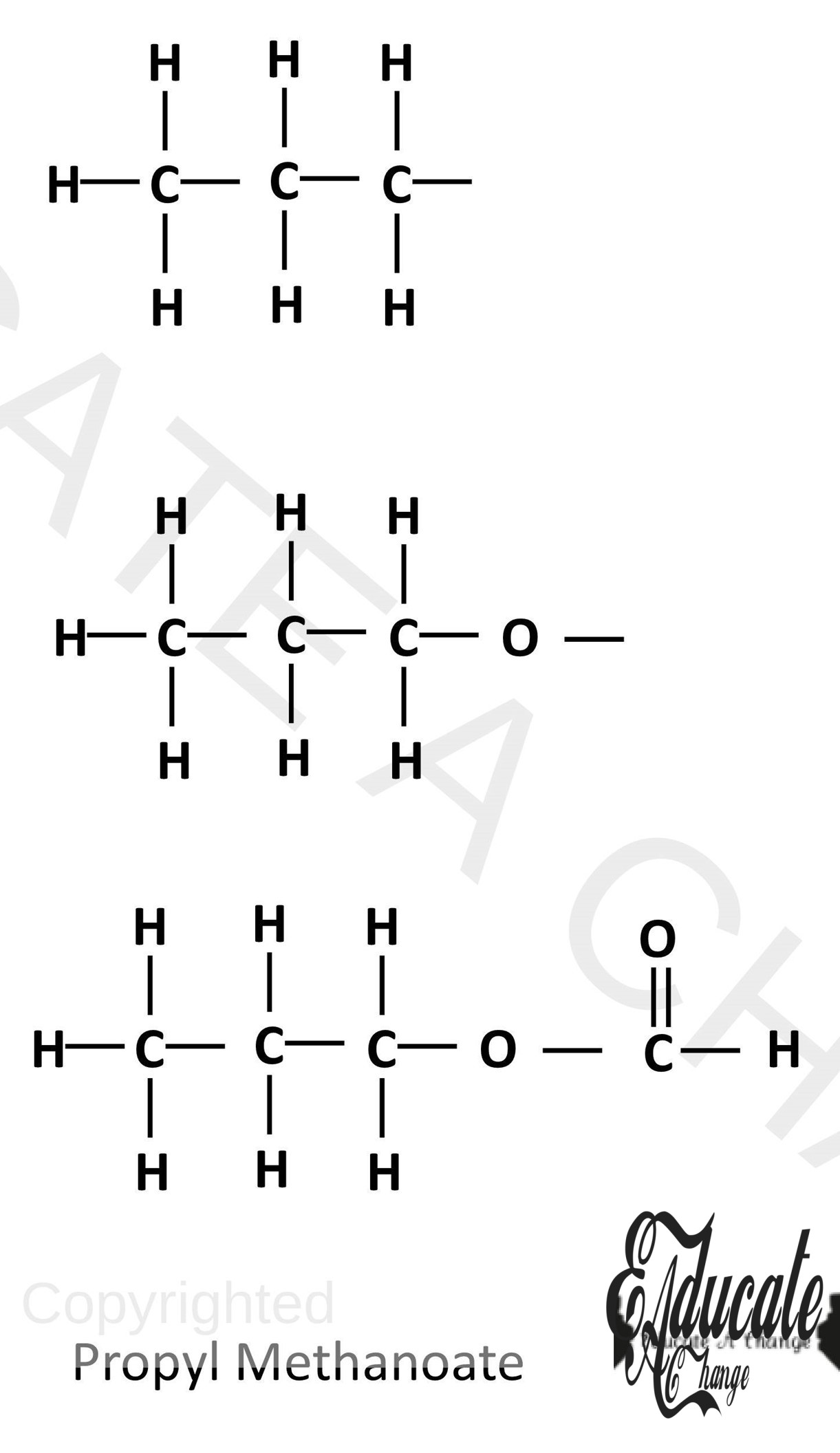
Drawing structures of esters:
For example we have to draw the structure of Propyl methanoate:
- Draw the alkyl, in this case Propyl first,
- Add an -O- for the alcohol and acid joining part
- Draw the carboxylate part. In this case, methyl with the structure meaning only OCH.
Topics:
- General
- Organic chemistry
- Homologous Series
- Common trends in Homologous series
- Hydrocarbons
- Saturated and Unsaturated Hydrocarbons
- General Carbon Rule
- Types of Reactions
- Combustion of Hydrocarbons
- Alkanes
- Alkanes
- Drawing Unbranched Alkanes given carbon number
- General Formula for Alkanes
- Physical Properties
- Chemical Properties
- Combustion
- Branched alkanes and Isomerism
- Alkenes
- Alkenes
- Drawing unbranched Alkenes given carbon number
- General formula for Alkenes
- Preparation of Alkenes
- Chemical Properties
- Combustion
- Branched alkenes and Isomerism
- Check for saturation
- Alkene Polymerization
- Margarine and vegetable oils
General
Organic Chemistry:
Organic chemistry deals with several homologous series of compounds that contain carbon. These include Alkanes, Alkenes, Alkyls, Alcohols and Carboxylic Acids.
Homologous Series:
Family of compounds with the same general formula which have similar chemical properties.
Common Trends in Homologous Series:
Viscosity:
- Viscosity is the internal resistance to the flow of a liquid. The thicker the liquid, the more the viscosity resulting in less flow.
- Viscosity of homologous organic series increase as carbon number increases.
Inflammability:
- Since the gaseous character decreases with the increase of carbon, the increase in carbon number decreases inflammability in organic homologous series.
Physical Properties:
- As the carbon number change in the homologous series, their properties change.
- More carbon means the melting and boiling point will also be higher hence a transition from gaseous state to liquid.
Hydrocarbons:
- Hydrocarbons are compounds that contain only carbon and hydrogen. Alkanes and Alkenes are hydrocarbons.
- The naphtha fraction from crude oil is the main source of hydrocarbons used as the feedstock for making a wide range of organic compound
Saturated and Unsaturated Hydrocarbons:
- Hydrocarbons that contain only single carbon-carbon or C-C bonds are known as saturated hydrocarbons.
- Hydrocarbons that contain double or triple C-C bonds (C=C and C≡C) are called unsaturated hydrocarbons.
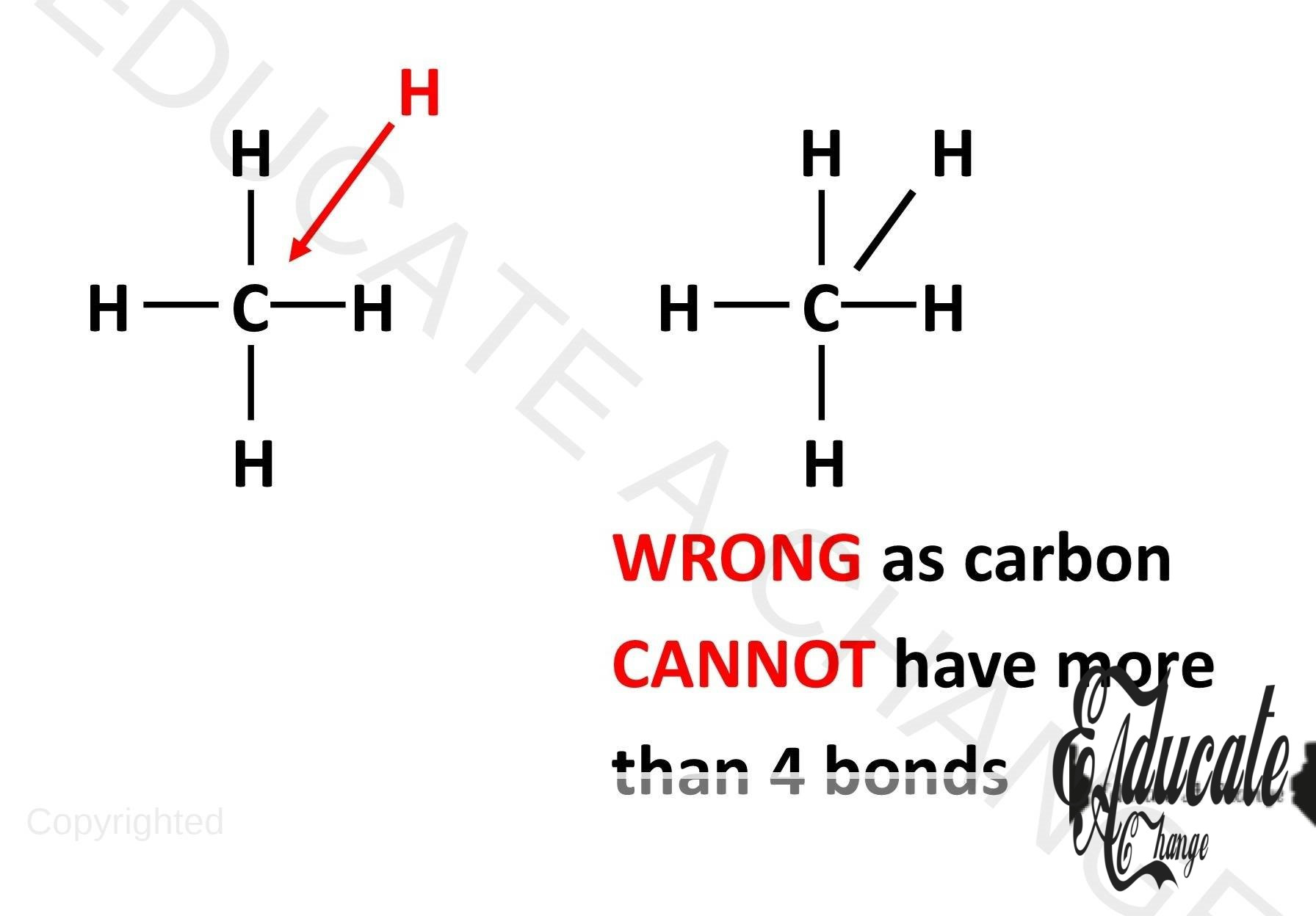
General Carbon Rule:
The MOST IMPORTANT rule in organic chemistry and when representing organic compounds is that CARBON CANNOT FORM MORE THAN 4 BONDS. The entire organic chemistry revolves around this rule so make sure whenever you are drawing any diagram, do count the number of carbon bonds for each and every carbon atom in the diagram to avoid any mistakes.
Types of Reactions:
Organic Compounds generally follow two types of reactions:
Addition Reaction:
Addition reaction takes place when some new element is added to an organic compound and nothing is removed. This can only work if there are double or triple covalent bonds. The bonds break to cater for the new addition.
Substitution Reaction/ Displacement Reaction:
When an existing element is removed, and another takes its place in a compound. If an organic compound is saturated, this is the only type of reaction that is possible.
Combustion of Hydrocarbons:
ALL hydrocarbons burn to make carbon dioxide and water.
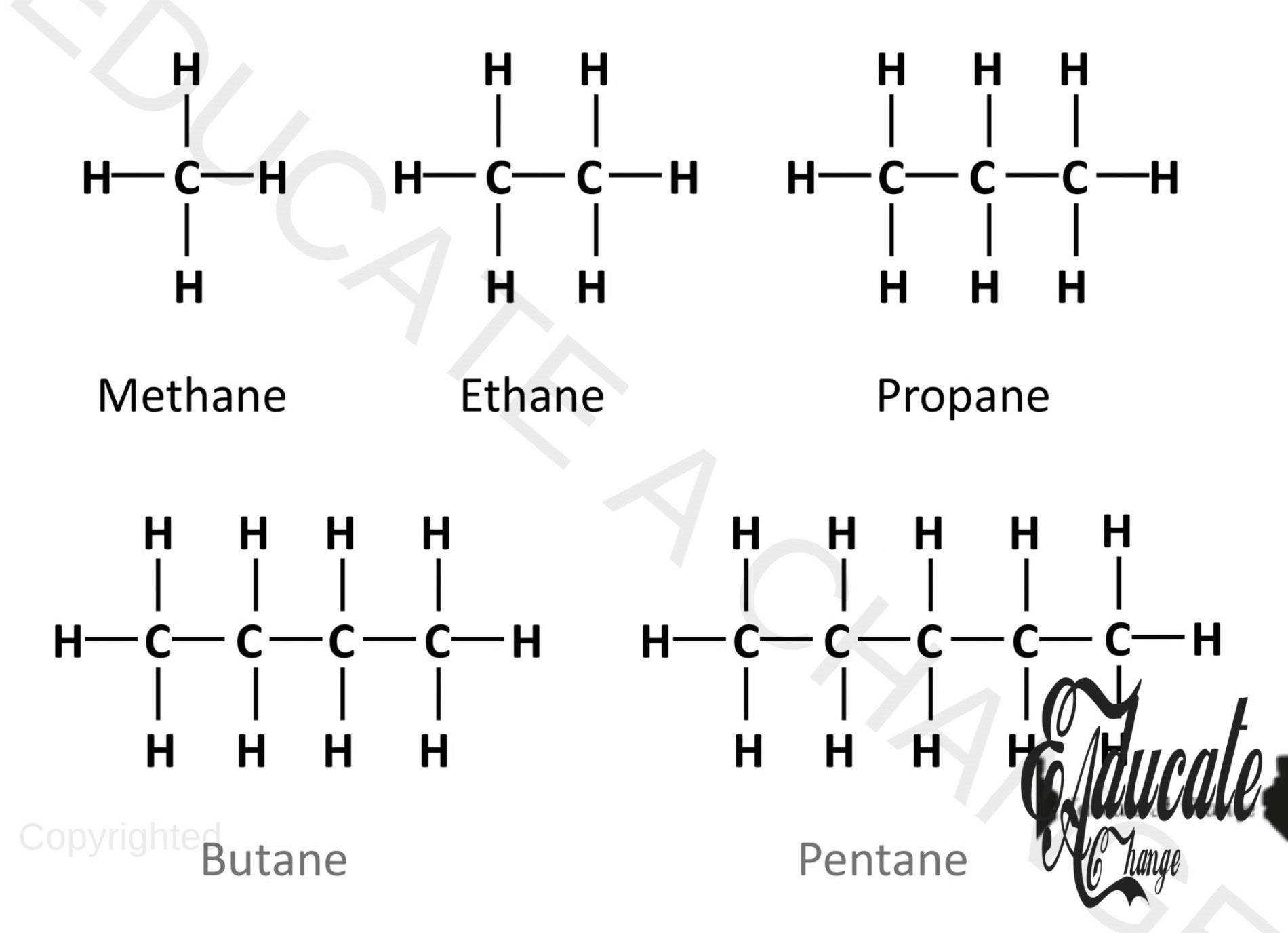
Alkanes:
Alkanes:
- Alkanes are homologous series of organic hydrocarbons that contain single covalent bonds.
- They are saturated hydrocarbons.
- The series of alkanes start from one carbon and goes on: Methane, ethane, propane, butane and so on with methane having only one carbon atom.
- As you can see, every carbon has four bonds in accordance with our rule.
- These types of alkanes are called unbranched alkanes where carbon is connected in a straight chain. All the names are written as n-alkane e.g. n-methane, n-butane etc. Every carbon is almost bonded with 2 other carbons in unbranched alkanes.
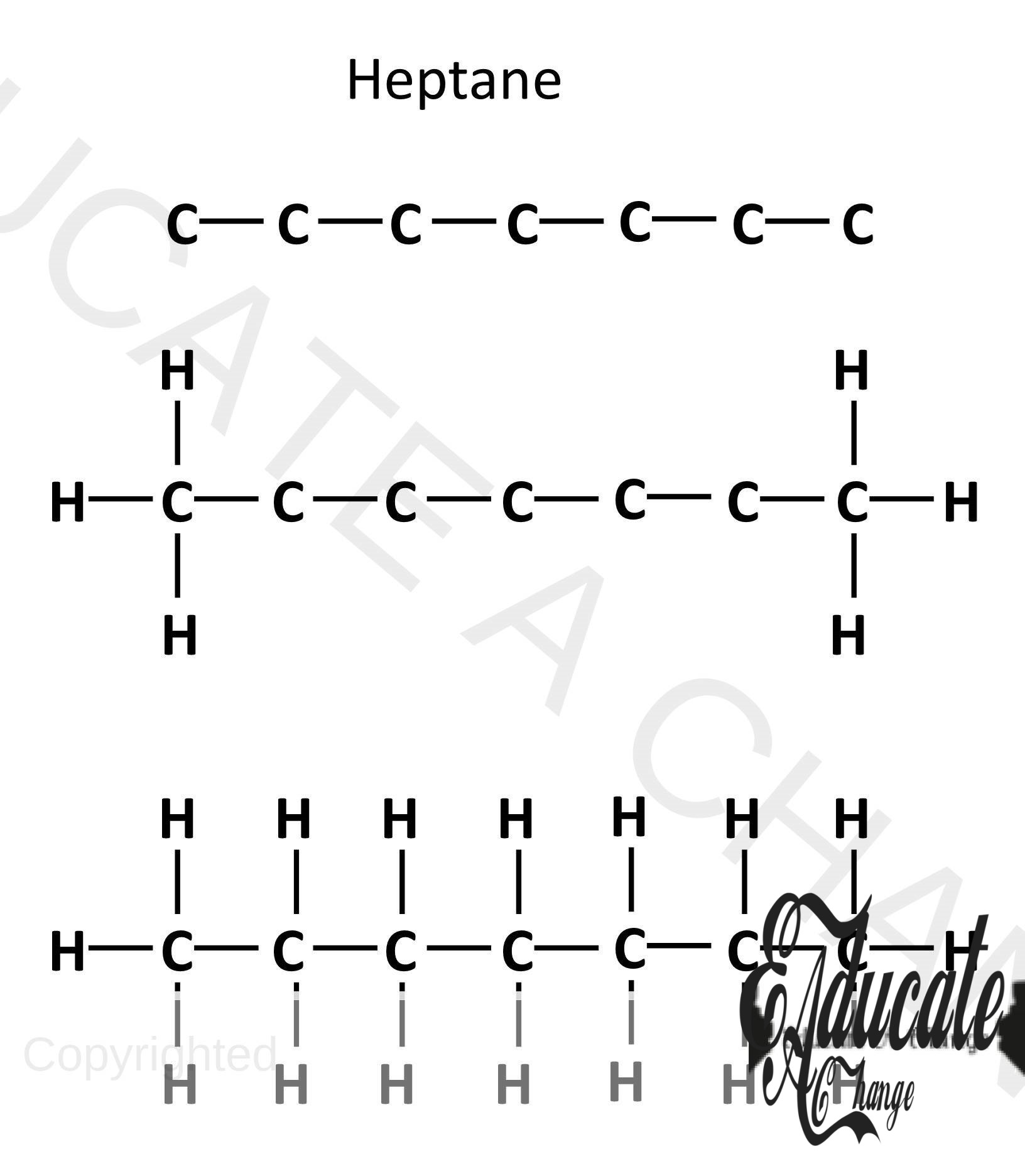
Drawing Unbranched Alkanes given carbon number:
- If we are to make an alkane with 7 carbons:
- We start by adding 7 carbons in a chain (C-C-C-C-C-C-C),
- The corner carbons will have 3 hydrogens each since they already have a bond with one carbon adjacent to them (C-C-)
- All the other carbons in the chain will have 2 hydrogens as they have two bonds with two adjacent carbons. (-C-)
- 7 carbon Alkane is called Heptane. (like heptagon with 7 sides)
General Formula for Alkanes:
A general formula is a way to determine the atoms of an alkane based on the number of carbon present. For alkanes: CnH2n + 2 For example: if the carbon number is 5 i.e. Pentane, we shall have C5H2(5) + 2 meaning C5H12. This can be verified from the above diagram as well.
Physical Properties:
C1 – C5 are gases, C6 – C17 are liquids and further they turn towards solid states or as thick pastes.
Chemical Properties:
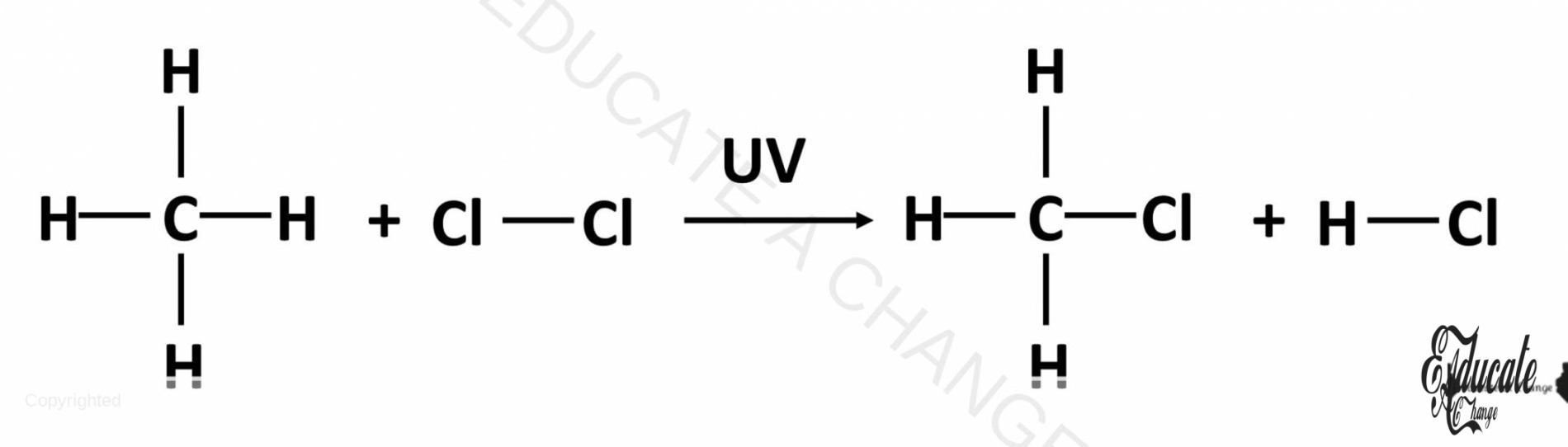
Since alkanes are saturated Hydrocarbons, they can only undergo substitution reactions. They cannot undergo addition reactions since there is no space to add any additional atoms to an alkane. That case would result in more than 4 bonds for carbon.
Alkanes undergo substitution reactions with, for example, halogens to create organic salts. For alkanes, since they are very unreactive, they only react with Chlorine mostly. This is called the halogenation reaction and required UV light. E.g. CH4 + Cl → CH3Cl + HCl (chloromethane or methyl chloride)
Combustion:
Since alkanes are hydrocarbons, they burn in oxygen to give water and Carbon dioxide. E.g. 2C2H6 + 7O2 → 4CO2 + 6H2O
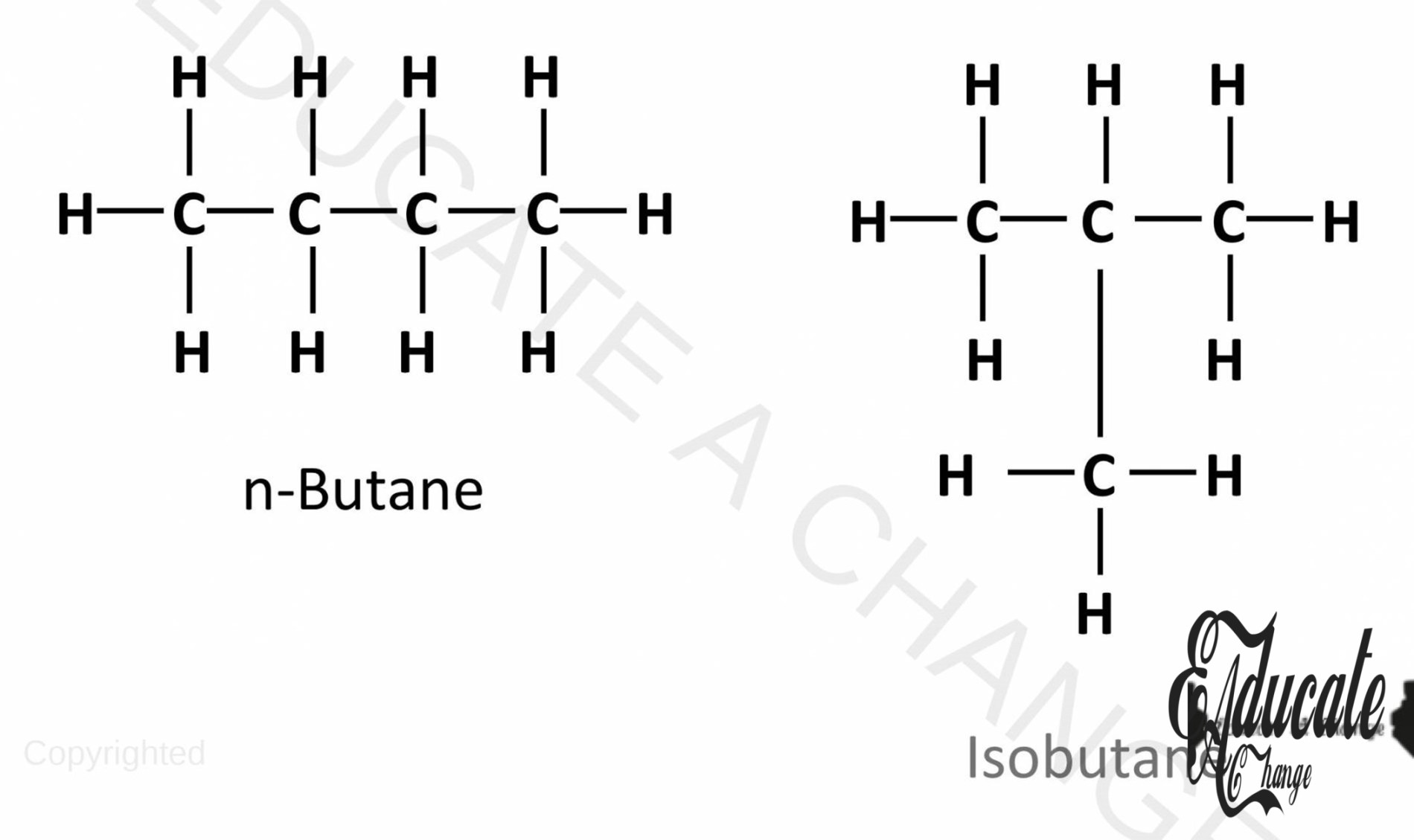
Branched Alkanes/ Isomerism:
- When in a straight chain of carbon, another sub-branch of carbon is added, that type of alkane is called branched alkane. Such type of alkanes is called isomers. The general formula still holds but the structure of the alkane changes.
- DO NOT CONFUSE ISOMERS WITH ISOTOPES STUDIED IN EARLIER CHAPTERS
- For the first three alkanes, there are no possible isomers.
- For butane, there are two isomers possible, isobutane and n-butane. The structure of isobutane is given below:
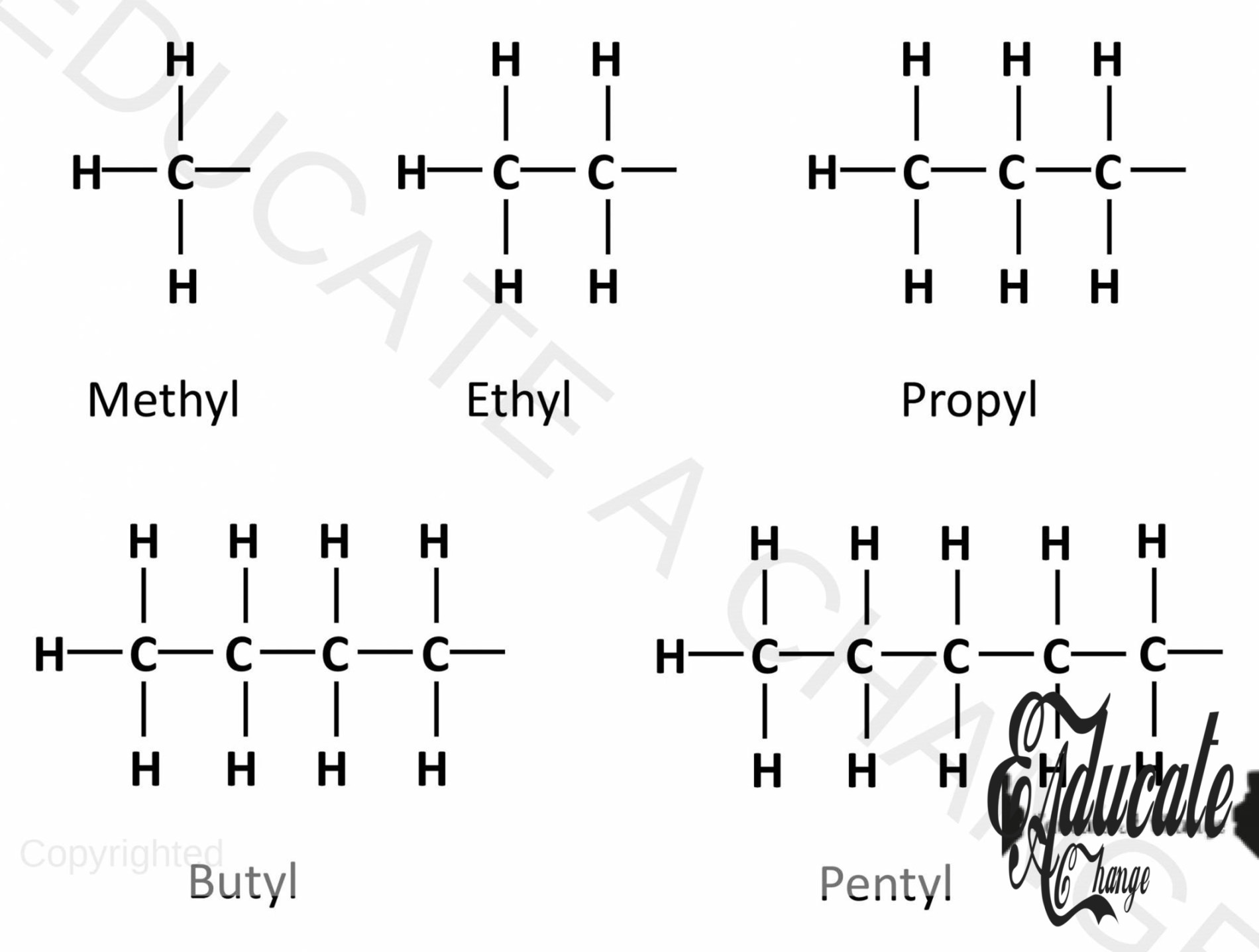
- Here, instead of having a straight chain, we have propane with a sub methane branch on the second carbon. We can call this 2-methylpropane, since methane is added on the 2nd carbon of propane.
- Methyl means methane with one less hydrogen and an extra bond: CH3- . These are called Alkyls. Such as Ethyl will be CH3-CH2-
- In order to identify isomers, count all the atoms and check if the number of atoms is same in both compounds.
Alkene:
Alkene:
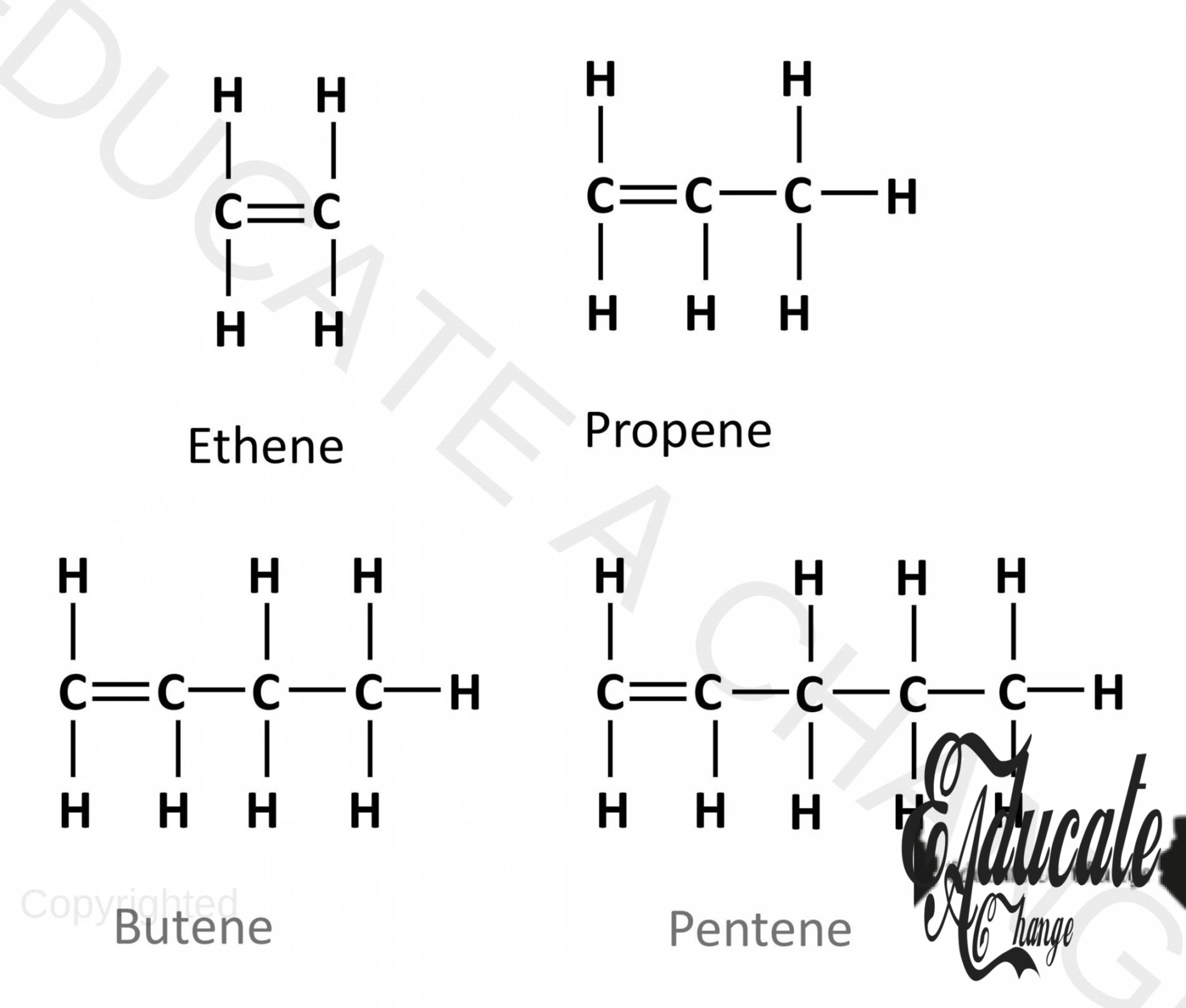
- Alkenes are a homologous series of unsaturated hydrocarbons containing double bonds.
- Alkene series Start with Ethene and goes on since we cannot have double bond with hydrogen in methane (doesn’t exist)
Drawing Unbranched Alkenes given carbon number:
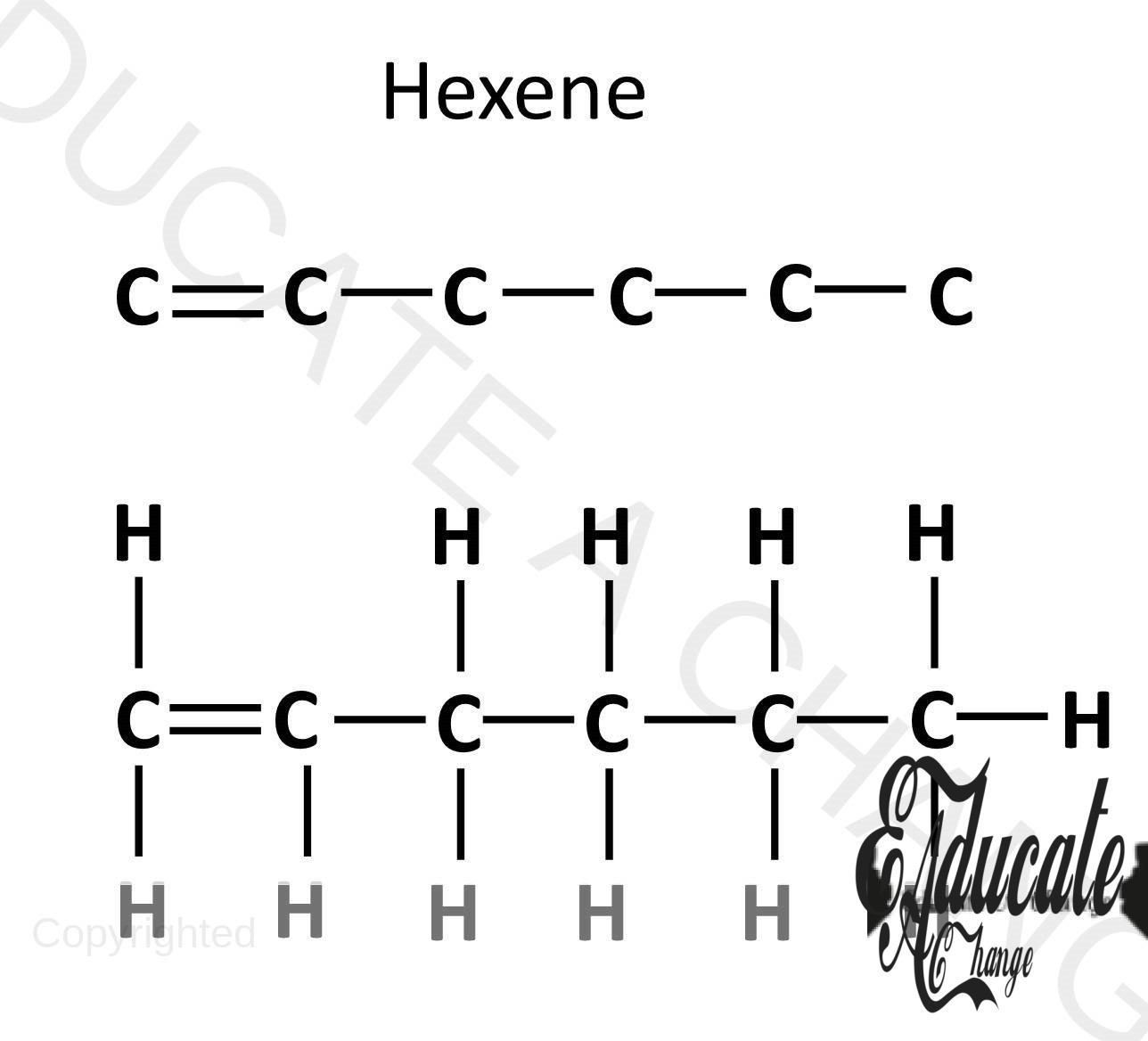
- If we are to make an alkene with 6 carbons:
- We start by adding 6 carbons in a chain with double bonds between first two carbon(C=C-C-C-C-C),
- Complete 4 bonds with all carbon and add hydrogens.
- 6 carbon Alkene is called Hexene. (like hexagon with 7 sides)
General Formula for Alkenes:
A general formula is a way to determine the atoms of an alkene based on the number of carbon present. For alkenes: CnH2n For example: if the carbon number is 5 i.e. Pentene, we shall have C5H2(5) meaning C5H10. This can be verified from the above diagram as well.
Preparation of Alkenes:
Alkenes can be obtained by two main methods:
Cracking of larger alkanes:
- Cracking is a process in which a larger hydrocarbon is broken into smaller useful fragments by heat.
- For example: C18H38 → C8H18 + 5C2H4 (octane and ethene)
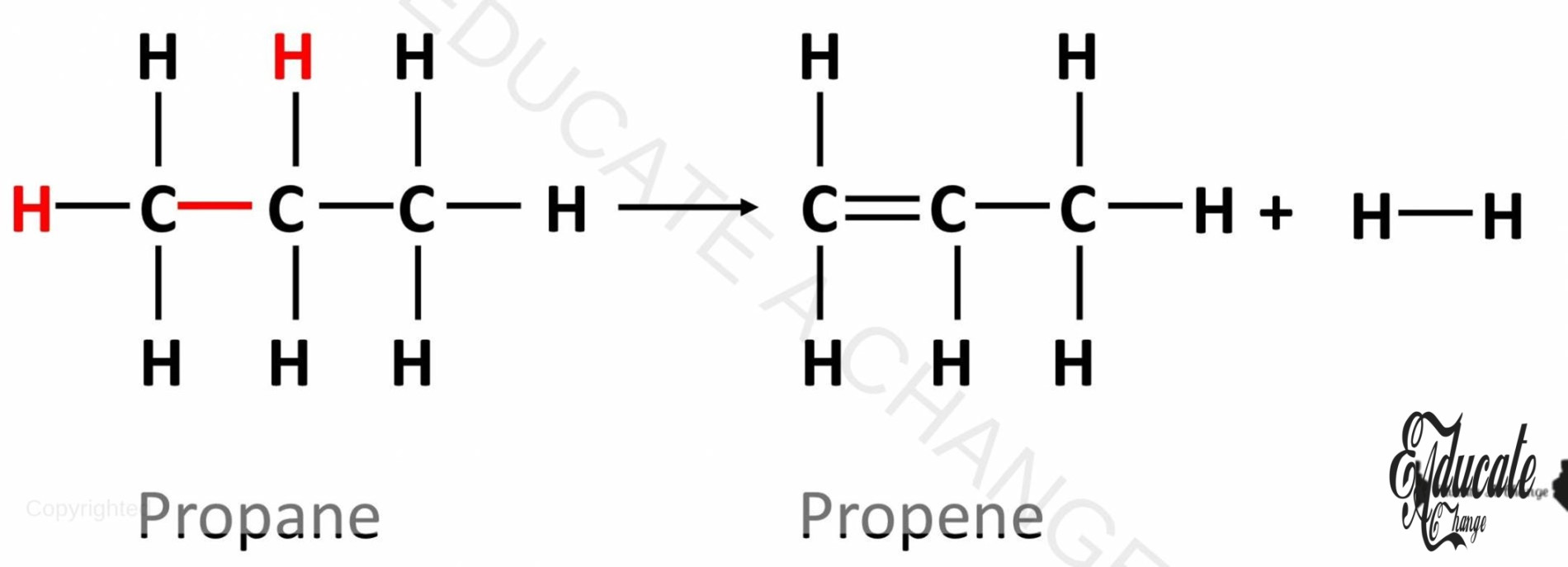
Dehydrogenation of Alkanes:
Dehydrogenation is a process in which we remove hydrogen from alkanes to turn them into alkenes. Dehydrogenation requires Nickel as catalyst. For example: the diagram shows dehydrogenation of propane into propene.
Chemical Properties:
Alkenes are unsaturated hydrocarbons and hence, they can undergo both addition and substitution reactions.
Addition reactions:
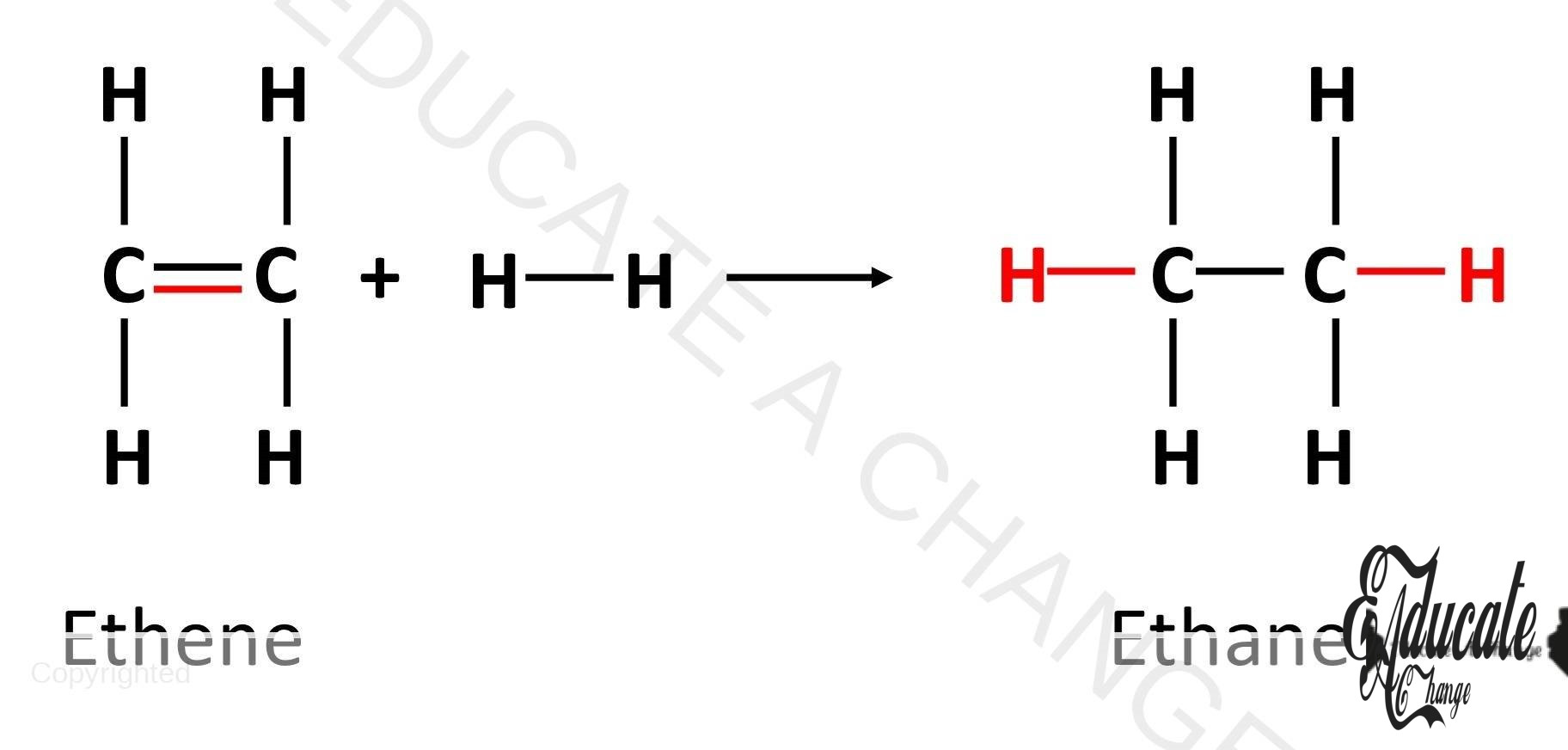
Hydrogenation: Alkenes are converted to alkanes in hydrogenation. The double bond is broken, and hydrogen is added to form alkane. For example: CH2=CH2 + H2 → CH2-CH2
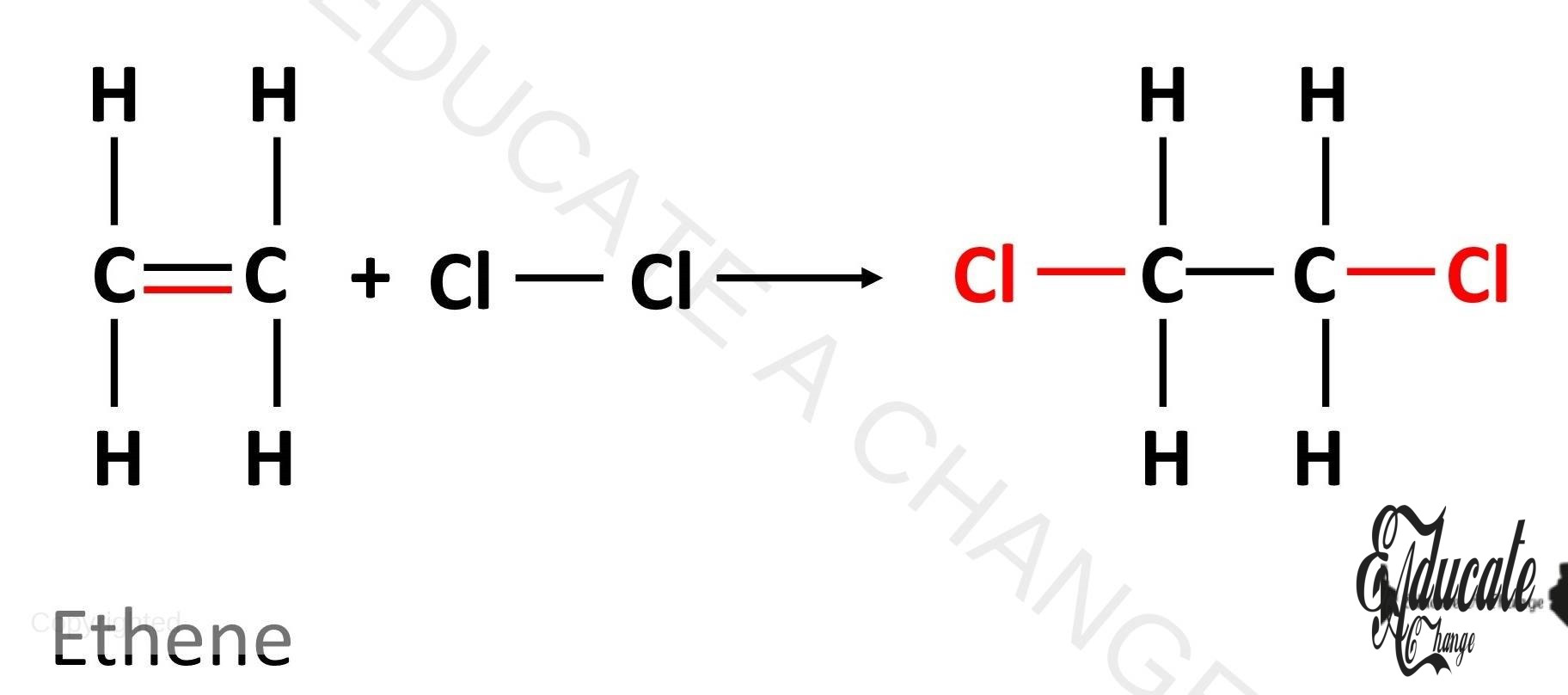
Halogenation: The addition of halogens in alkenes result in the making of organic salts. The double bond is broken and each carbon atom with the bond gets one halogen atom. For example: CH2=CH2 + Cl2 → CH2Cl – CH2Cl
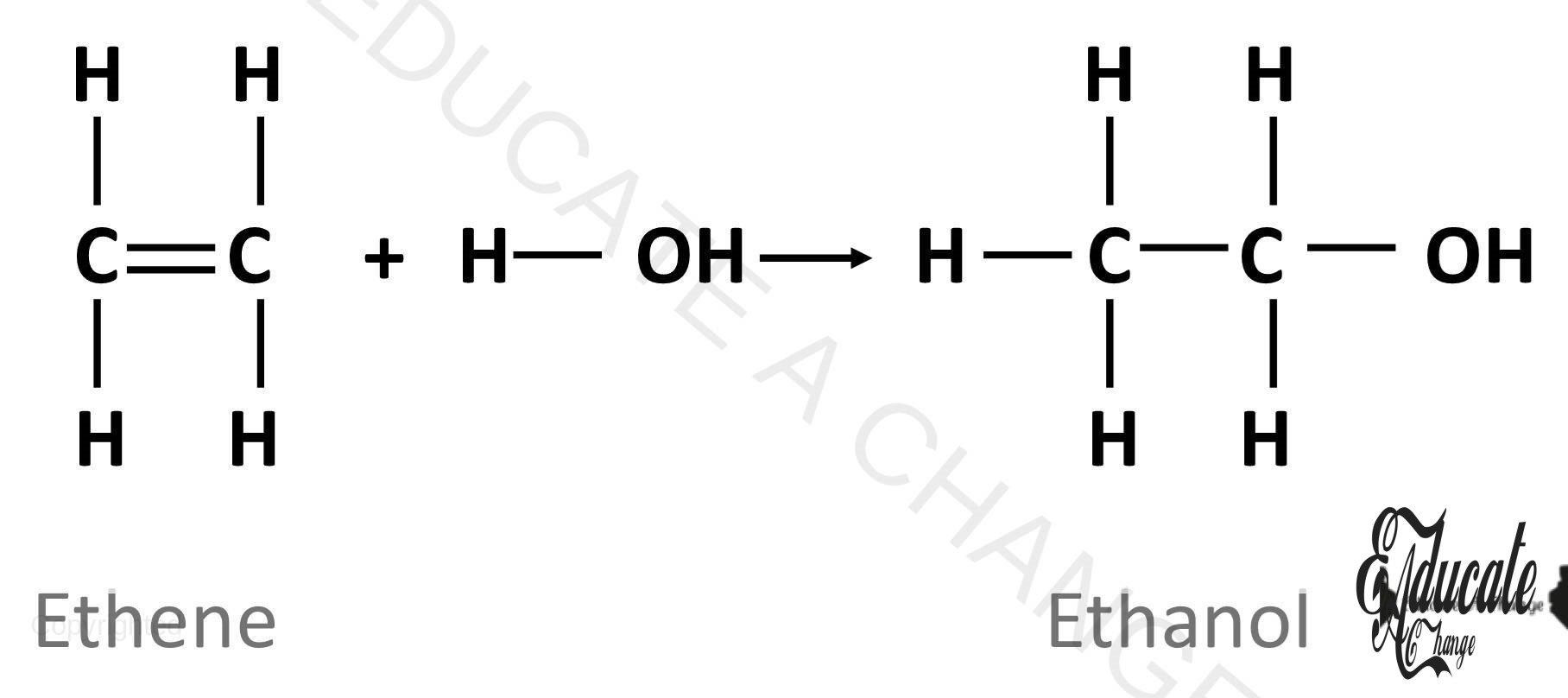
Reaction with water: Alkenes are organic compounds and organic compounds naturally react with organic substances. However, in the presence of hydrochloric acid or sulfuric acid as catalyst, alkenes react with water to form alcohols. Alcohols have OH and will be discussed in next section. e.g. CH2=CH2 + H2O → CH3-CH2OH (ethanol)
Combustion:
Since alkenes are hydrocarbons, they burn in oxygen to give water and Carbon dioxide. E.g. C2H4 + 3O2 → 2CO2 + 2H2O
Branched alkenes and Isomerism:
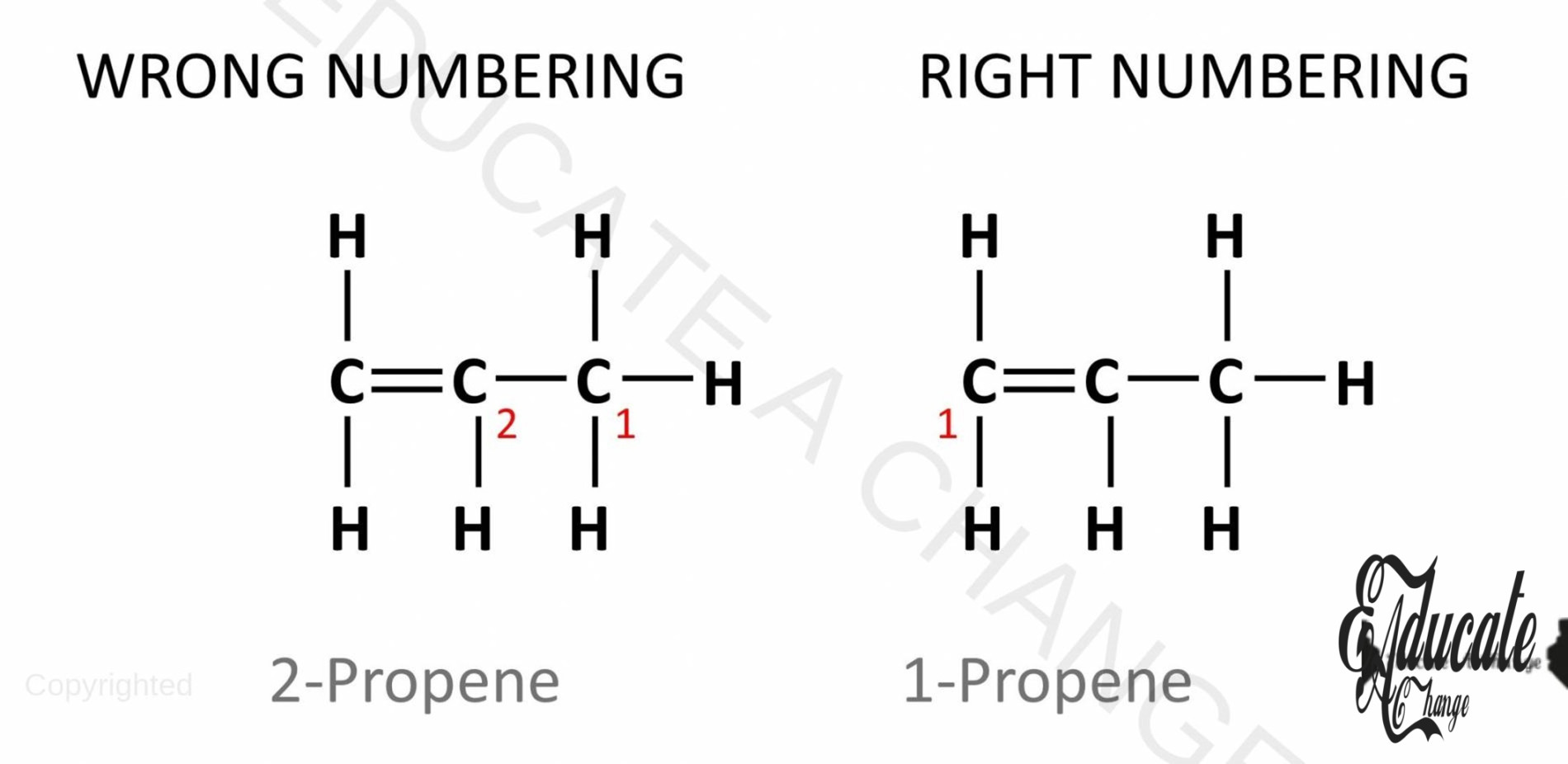
- In alkenes, the carbon atom where the double bond is present represents the number with the alkene. For example, all the above unbranched alkenes shown had double bond at the first atom. Hence, we call them 1-alkene or 1-ethene, 1-propene etc.
- Remember to always give numbers that are the lowest, i.e. start from the side where the double bond is closest. This means that instead of calling it 2-propene, we call it 1-propene.
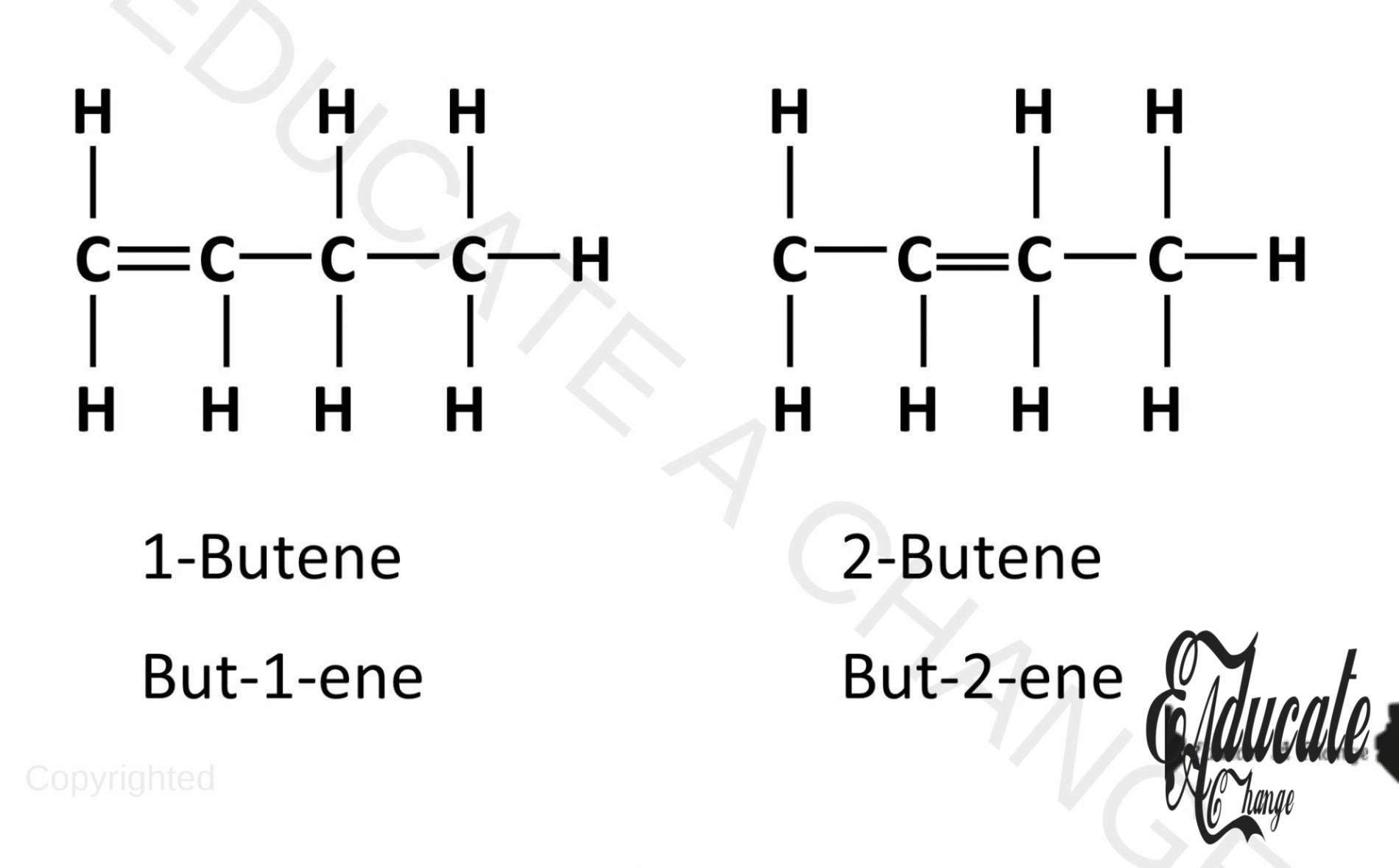
- Firstly, it is possible to have unbranched isomers in alkenes based on the placement of double bonds. Examples of such isomers are shown below.
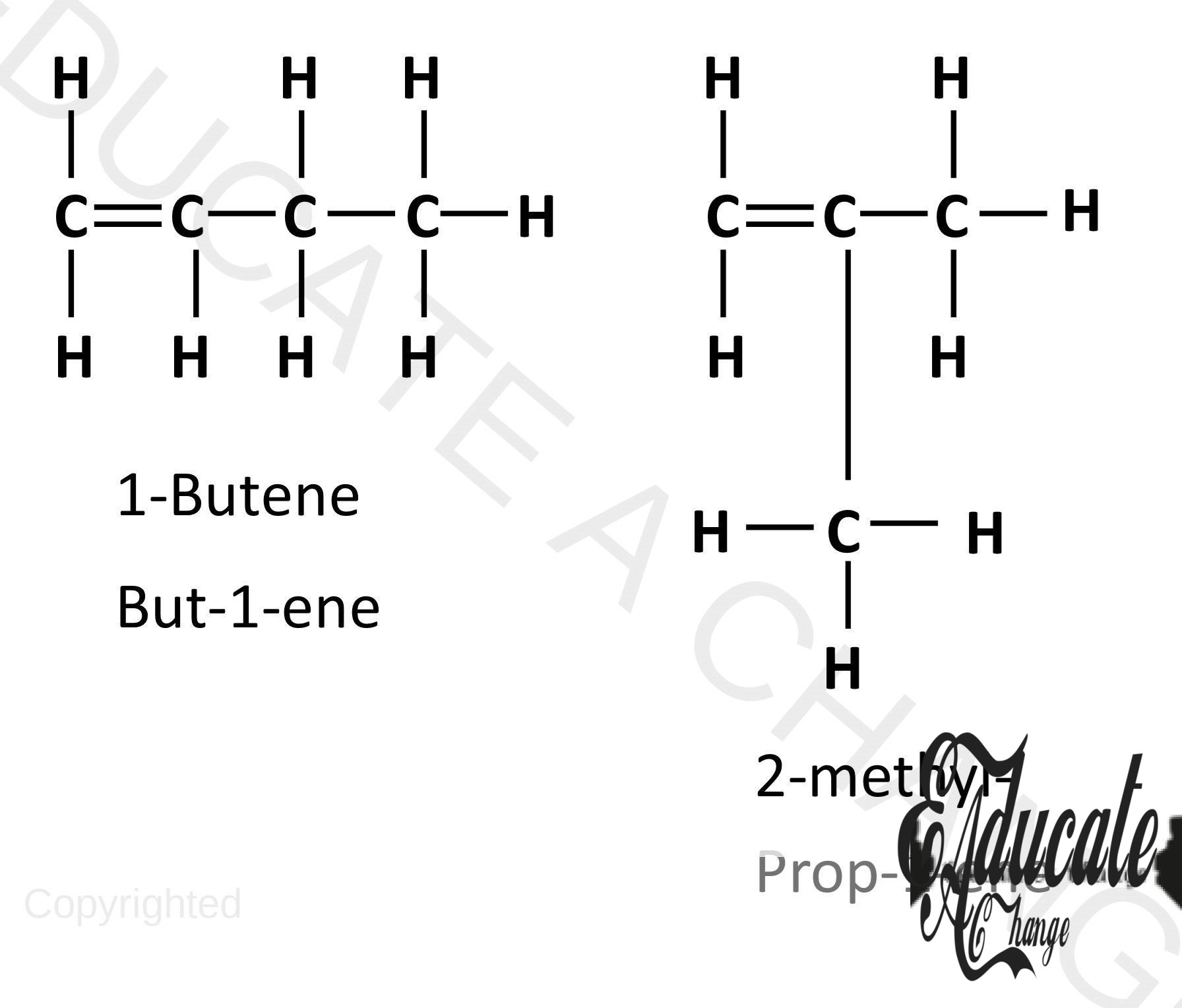
- Alkenes also have branched isomers that start from butene.
Check for saturation:
In order to check whether a substance is a saturated hydrocarbon or not, we add bromine to it. Since bromine has a reddish-brown color, if it undergoes halogenation, the reddish color fades to colorless.
Alkene Polymerization:
Poly means many. Polymers are long chain compounds that have many small monomers or units. Alkenes have to ability to make polymer chains. This happens when the double bonds of alkene break down and another similar alkene is added to the broken bond. We name then as poly alkene e.g. poly ethene.
Margarine and Vegetable Oils:
Polyunsaturated compounds are compounds that have many double or triple bonds. Such a compound is vegetable oil. Vegetable oil is converted to margarine by the addition of hydrogen with breaks the double bonds. This makes a solid product i.e. margarine. For more such amazing notes of every subject, check out our courses page and shop section: