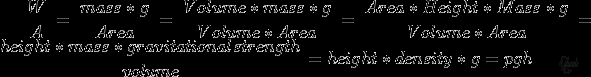Pressure and Energy Sources | O Level Physics 5054 & IGCSE Physics 0625 | Detailed Free Notes To Score An A Star (A*)
Lesson Objectives
- Pressure:
- Definition and Formula
- Everyday Applications
- Pressure in Liquids
- Barometer
- Manometer
- Hydraulic Systems
- Boyle’s Law
- Energy Sources and Transfer of Energy
- Forms of Energy
- Sources of Energy
- Principle of Conservation of Energy
- Kinetic and Potential Energy
- Work
- Efficiency
- Power
- Electricity Generation Block Diagram
- Environmental Issues with Power Generation:
Pressure:
Definition and Formula:
- Pressure is defined as force acting per unit area
- Pressure = force /area, so P=F/A
- SI Unit for pressure: Pascal, N/m2 (For solids)
- One pascal is the force of one newton acting on unit area at right angle.
- General Rule: always measure pressure from bottom to top.
Atmospheric Pressure:
Atmospheric pressure can be defined as pressure caused by the weight of air. Example:
- If a can is filled with hot water and then we dip it upside down in a tub that contains cold water, this will cause the can to crumble.
- This is because the molecule will slow down and the pressure from inside the can will be reduced as the can will cool down, resulting in a pressure mismatch.
- The higher pressure from outside will cause the can to crumble.
Everyday Applications:
There are numerous applications of pressure. We shall discuss a few to give you a better idea of how important the application of pressure is in our lives.
- Using a straw
- Something as simple as a straw uses the principles of pressure to work. When we suck a straw, the air particles inside a straw are taken in by us and this creates a vacuum without particles. The moving particles of the liquid put a higher pressure in the straw as compared to the particles that are left inside the straw, causing the drink to move up the straw which we get to drink.
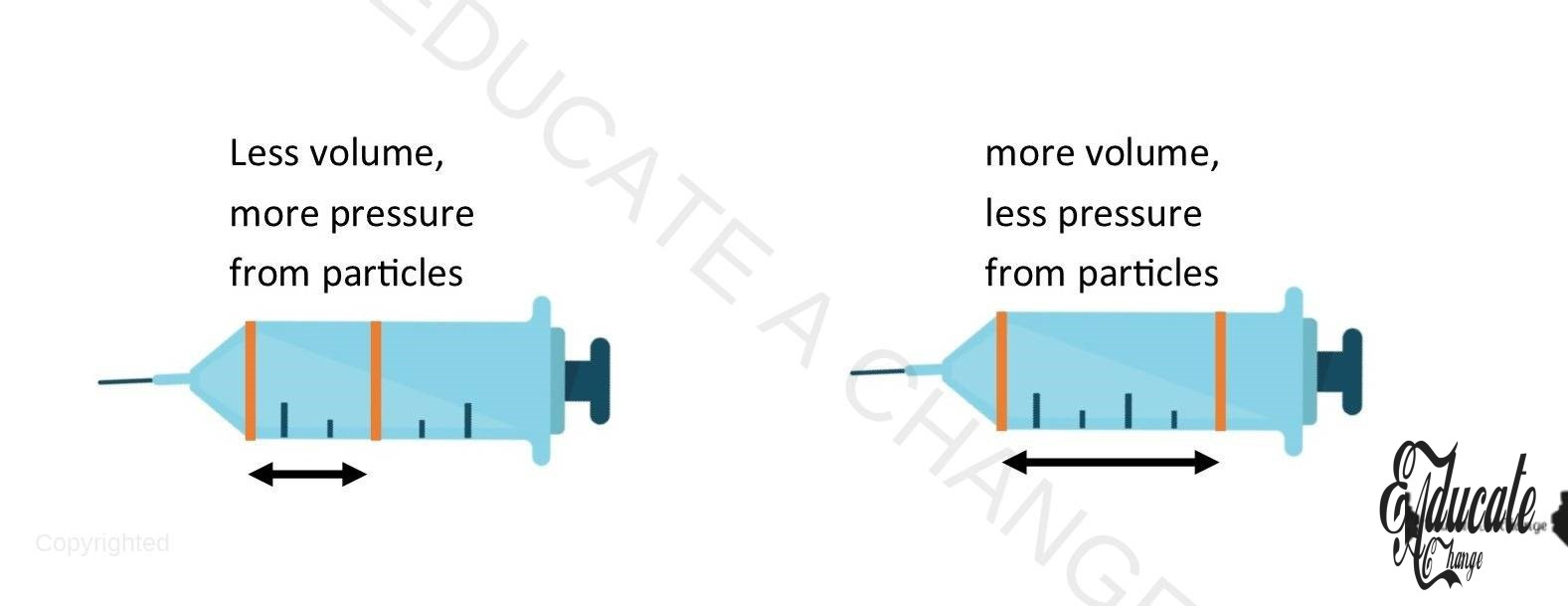
- Syringes
- When we place the syringe inside a medicine and pull the handle, the air particles inside the syringe get more volume and hence, their pressure decreases (discussed in Boyle’s Law). This causes the medicine to be drawn in since it places a higher pressure as compared to the particles inside the syringe.
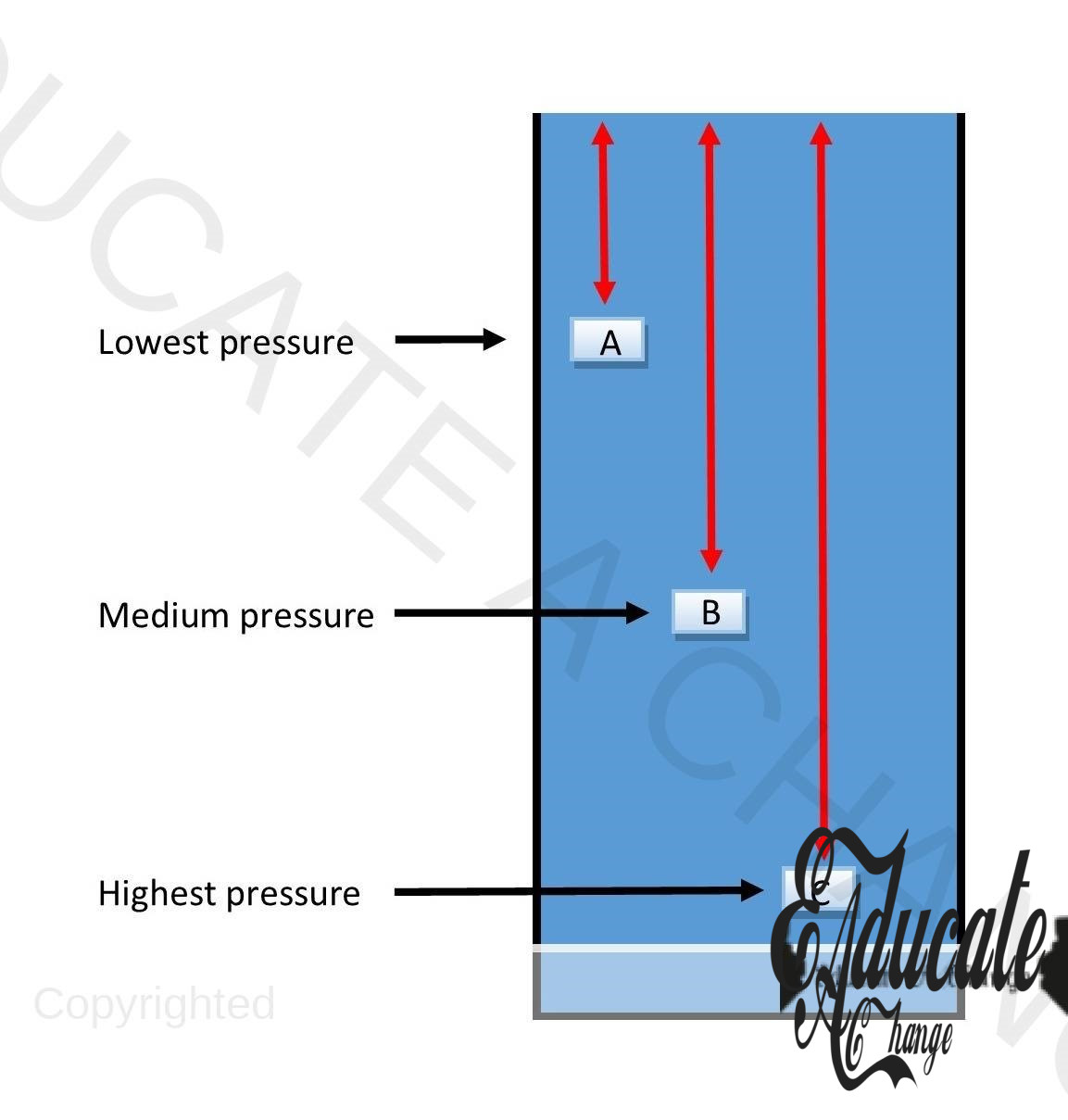
Pressure in Liquids:
In liquid columns, pressure increases with depth as the height increases (bottom to top) with increase in depth. Hence, the lowest part of the liquid column has the highest pressure.
- Consider the above liquid column. Three objects are at three different heights in the column A, B and C. As we can see by the red lines, the height from A to top is the lowest among the three, it means that object A has the least pressure being applied on it. C is at the most depth, so the pressure is highest there.
- In liquids, the force being applied is weight and hence pressure = weight/ area.
- weight = mass x g
- Pressure in Liquids= pgh
- Liquid pressure does not depend on base area.
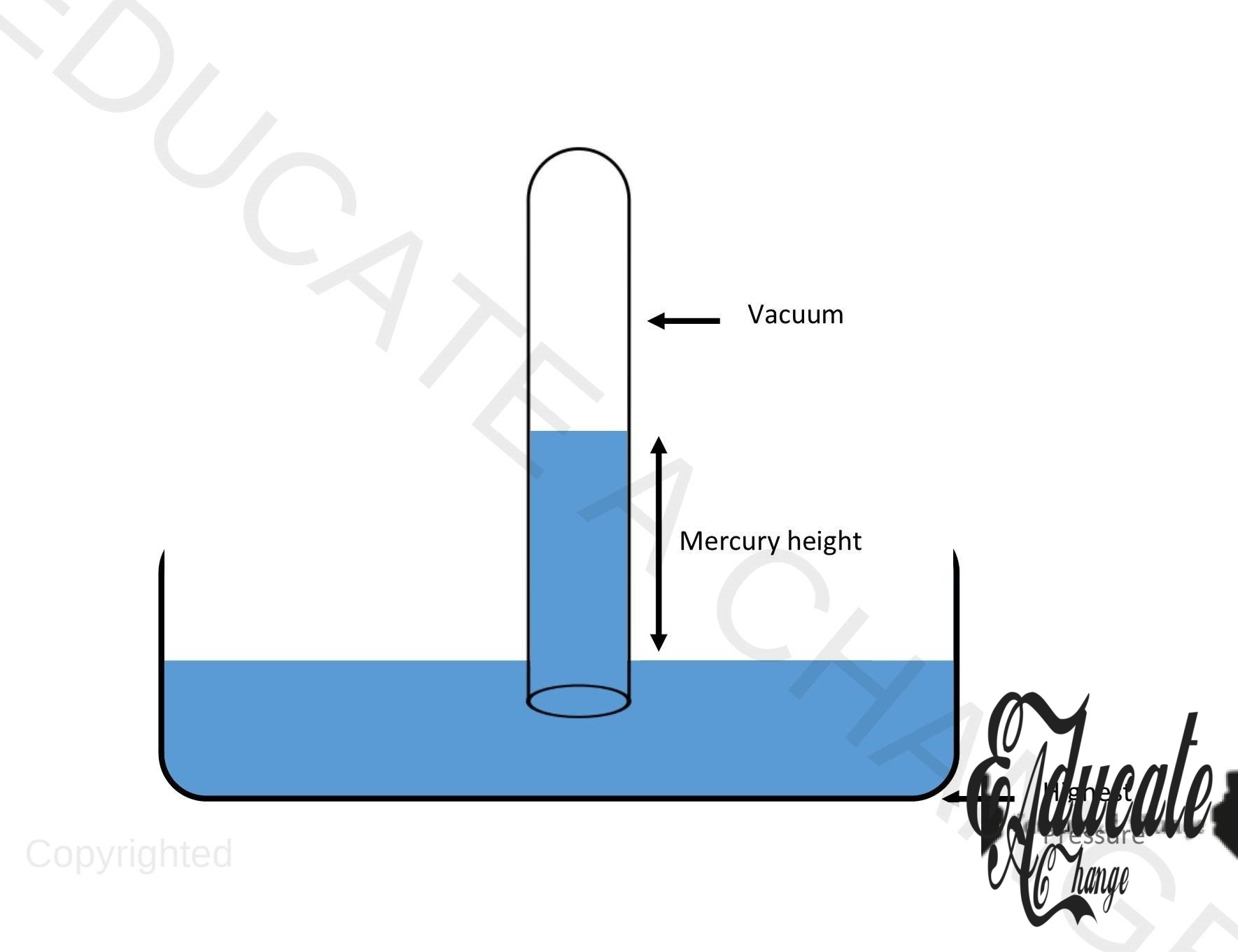
Barometer:
Barometer is an instrument used to measure air pressure. The diagram shows a mercury barometer.
- Our normal atmospheric pressure is 100000 pascal.
- Density of mercury is 13600 kg /m³ so it is approx. 13.5 times heavier than water. (Density of water is 1000 kg/m³)
- We do not use water in the barometer as water is lighter and would require a very large height to measure air pressure.
- The barometer works by balancing the air pressure with the pressure by mercury. As the pressure in the liquid tube is more, the mercury comes down until it equals the air pressure.
- We make measurements of height to calculate pressure using mercury’s density.
- Pressure for liquid formula is used for the calculations.
- Change in the area or diameter does not affect in liquids. (mercury)
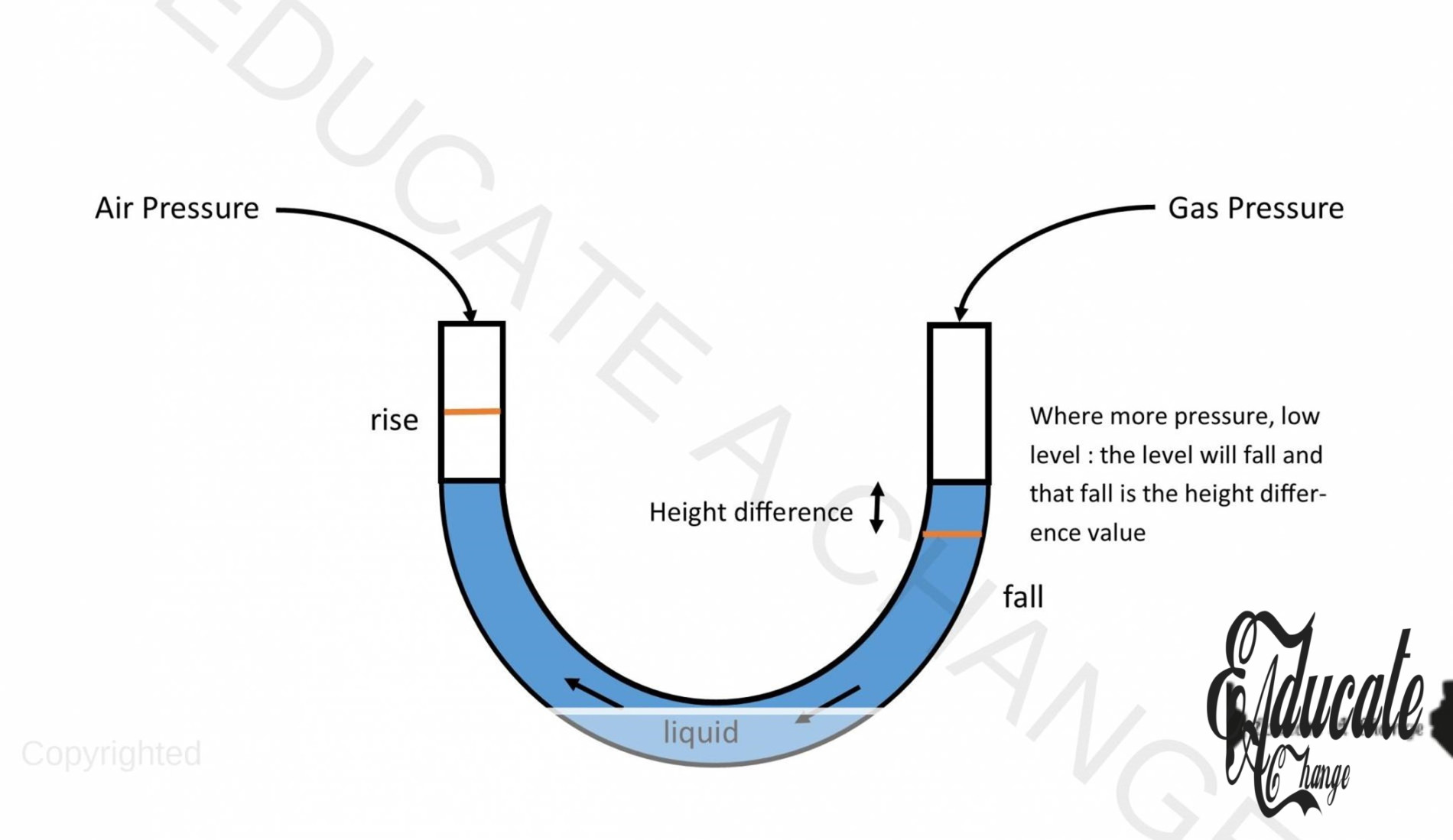
Manometer:
Manometer is an instrument that is used to measure gas pressure.
Working of Manometer:
- We attach our gas on one side of the manometer and the atmospheric pressure is applied on the other side of the manometer.
- The tube is filled with the liquid.
- Pressure of our gas = atmospheric pressure + difference in the levels of height
- Make sure to convert from cm to m
- If liquid in column of gas pressure rises, then its pressure is lower than the atmospheric pressure and then we would use: Atmospheric pressure – difference in liquid height.
- The side where there is more pressure, the level will be low, and the other side will rise.
- High density liquids have high pressure measuring capability.
- We decrease our density to increase the sensitivity of our manometer.
- High density liquid would be difficult to measure in low pressure of gas since their will be a slight change in height only.
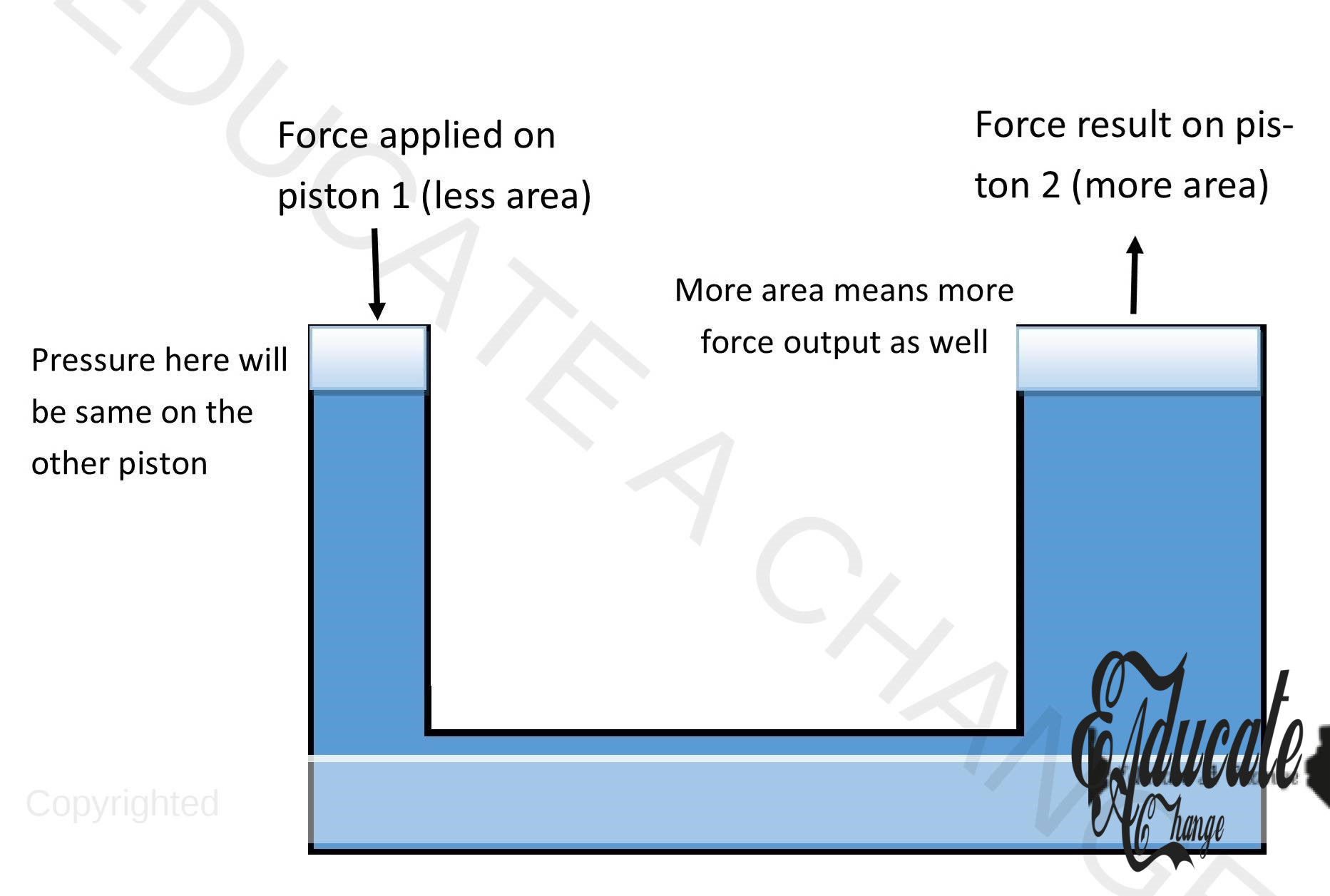
Hydraulic Systems:
- Hydraulic systems are systems where we apply force on one end and achieve a much bigger or equal force on the other end (by filling the middle of the system with an uncompressible liquid).
- These are used in brakes and heavy machinery.
- The hydraulic systems work with the principle that the pressure exerted on one end of the hydraulic system will be the same on the other end, if an uncompressible liquid is used in between.
- So:
- Hence, if we increase the area of the other piston, the force on that piston will also increase.
Boyle’s Law:
The law states that volume of a given mass of a gas inversely proportional to the pressure in constant temperature.
- V = k/p
- VP = K
- V1P1 =k and V2P2 = k
Hence:
- V1P1 = V2P2
Energy Sources and Transfer of Energy:
- Energy brings change in the world.
- Energy can be transferred from one place to another or converted from one form to another form.
- By definition, Energy is the ability to do work.
- SI UNIT: Joules/J
Forms of Energy:
There are various different forms of energy. The most important forms are:
- Chemical/fuel Energy
- Found in fuels such as oil, coal explosives etc.
- Nuclear Energy
- Made in nuclear reactor and is in atom bombs
- Radiant/Light Energy
- Energy of the electromagnetic spectrum (explained in later section)
- Electric Energy
- Energy that deals with the current, electric drills and electrical appliances
- Internal Energy (not usually considered)
- Energy that is possessed by atoms and molecules
- Mechanical Energy
- Potential and Kinetic Energy
Sources of Energy:
There are two major categories of energy sources:
- Fuel
- Coal, oil, gas (non-renewable energy source)
- Non-Fuel
- Water, solar, wind, nuclear (renewable energy source)
Chemical Energy:
- Stored in structure of atoms and molecules
- Produced by regrouping the structure of atoms
Hydroelectric Generators:
- Energy is stored in the water as potential energy
- As water flows, the energy is converted to kinetic energy
- The water spins the turbines and the turbines produce electricity
Solar Energy:
- The main source of energy is Sun.
- Solar energy is used to produce electricity through solar cells.
Nuclear Energy:
The two processes where nuclear energy can be obtained are:
- Nuclear Fission:
- The splitting of atoms of heavy elements into lighter atoms.
- Nuclear Fusion:
- It is the combination of atomic nuclei of light elements into heavy elements.
Wind Energy:
- Windmills are used to convert wind into electrical energy by turning the turbines which generate electricity
Geothermal Energy:
- Geothermal energy is the natural energy of the Earth. The energy comes from the center of the Earth, which has hotspot or molten rock.
- Cold water is subjected to the hot molten rock which generates steam and hence, can be used to generate electricity.
Principle of Conservation of Energy:
The principle of conservation of energy states that energy can neither be created nor destroyed: it can be converted from one form to another form or transferred from one place to another place, but the total energy remains constant.
Examples of Energy conversions showing principle of conservation:
- Diver diving on board:
- Stored chemical energy-> elastic PE-> KE
- Hammering a nail:
- GPE (raised hammer) ->KE ->sound
- Burning of coal:
- Chemical PE –> thermal energy/ light energy
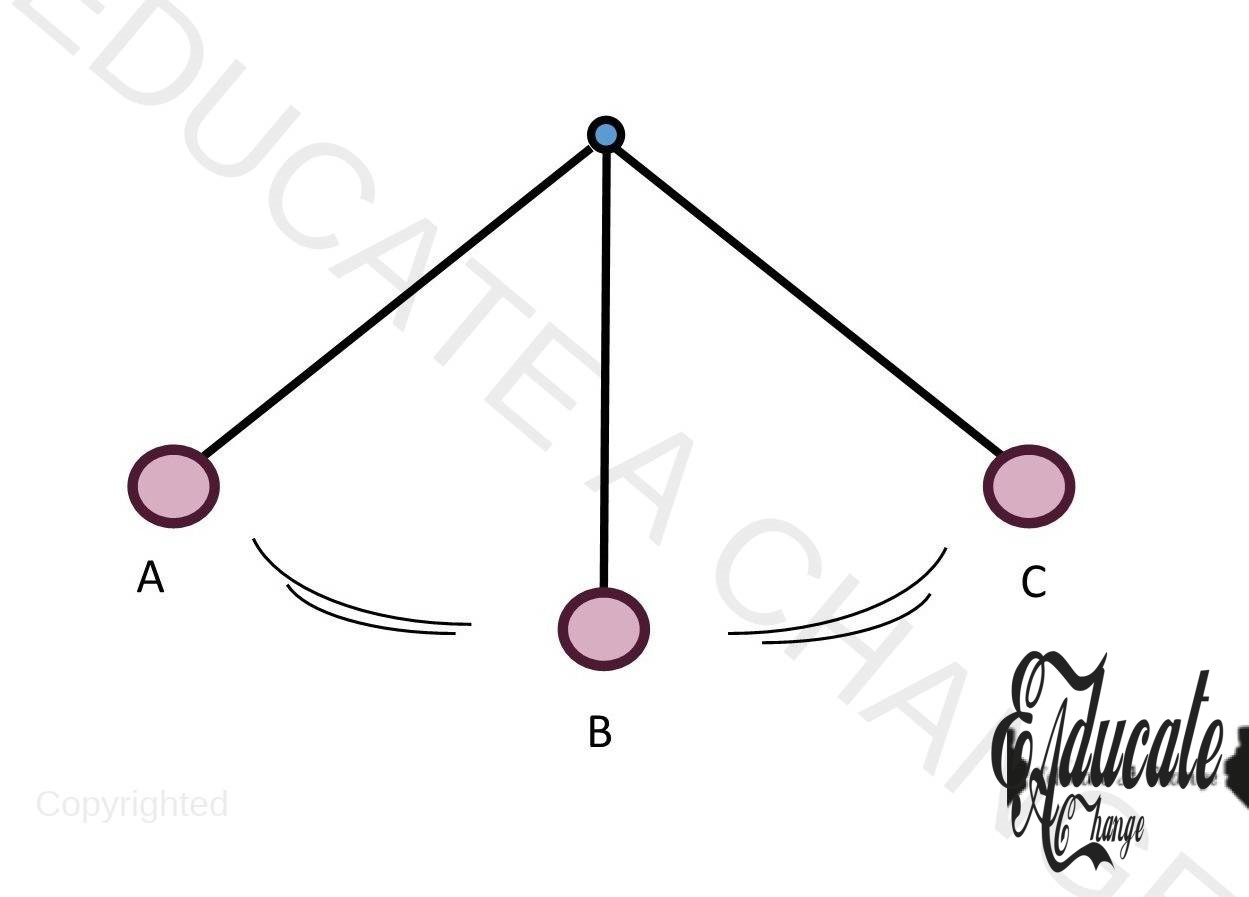
Principle of Conversation of Energy in an Ideal Pendulum:
Consider the diagram:
- At position A, pendulum would have GPE since it has been raised to a certain height
- From A to B, GPE will start decreasing and KE will increase as the pendulum moves towards the other end
- At B, the KE will be maximum.
- From B to C, the GPE will start to increase and KE will decrease.
- The cycle will continue with no change as in an ideal pendulum, there is no air resistance or friction. There will be conservation of energy in each cycle so the pendulum will keep on swinging forever.
Principle of Conversation of Energy in a Non-ideal Pendulum:
- Motion will be similar to an ideal pendulum.
- However, frictional forces will act and cause some energy to be converted to heat and get lost.
- The pendulum will slowly lose the height and come to rest at B.
- GPE = KE + thermal/heat energy
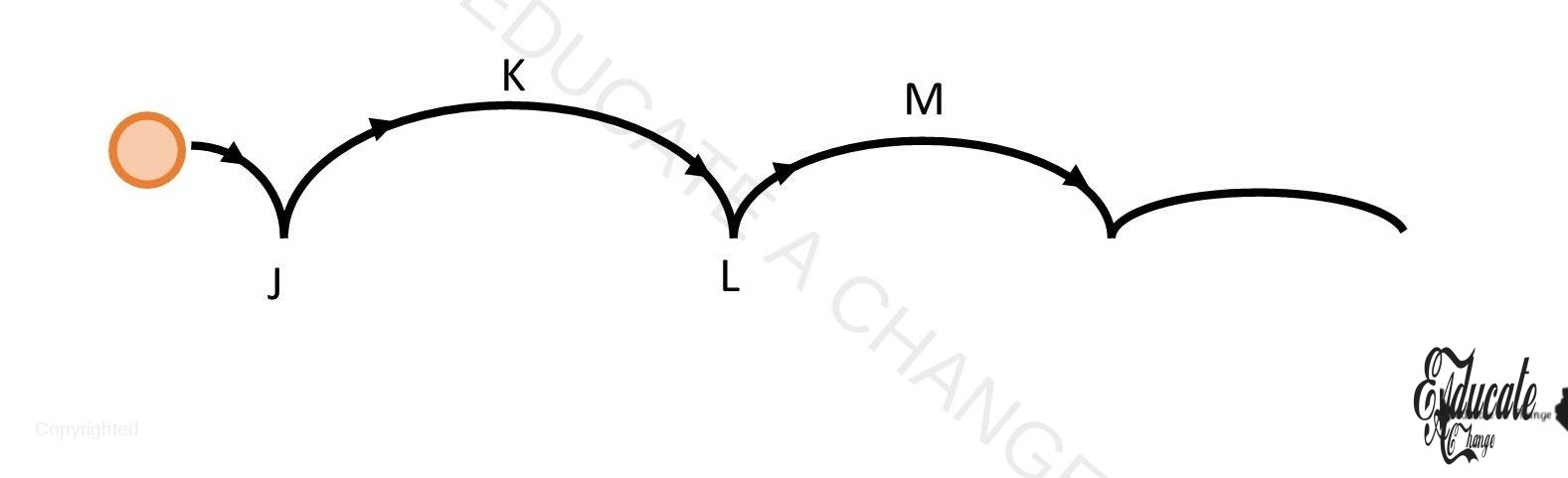
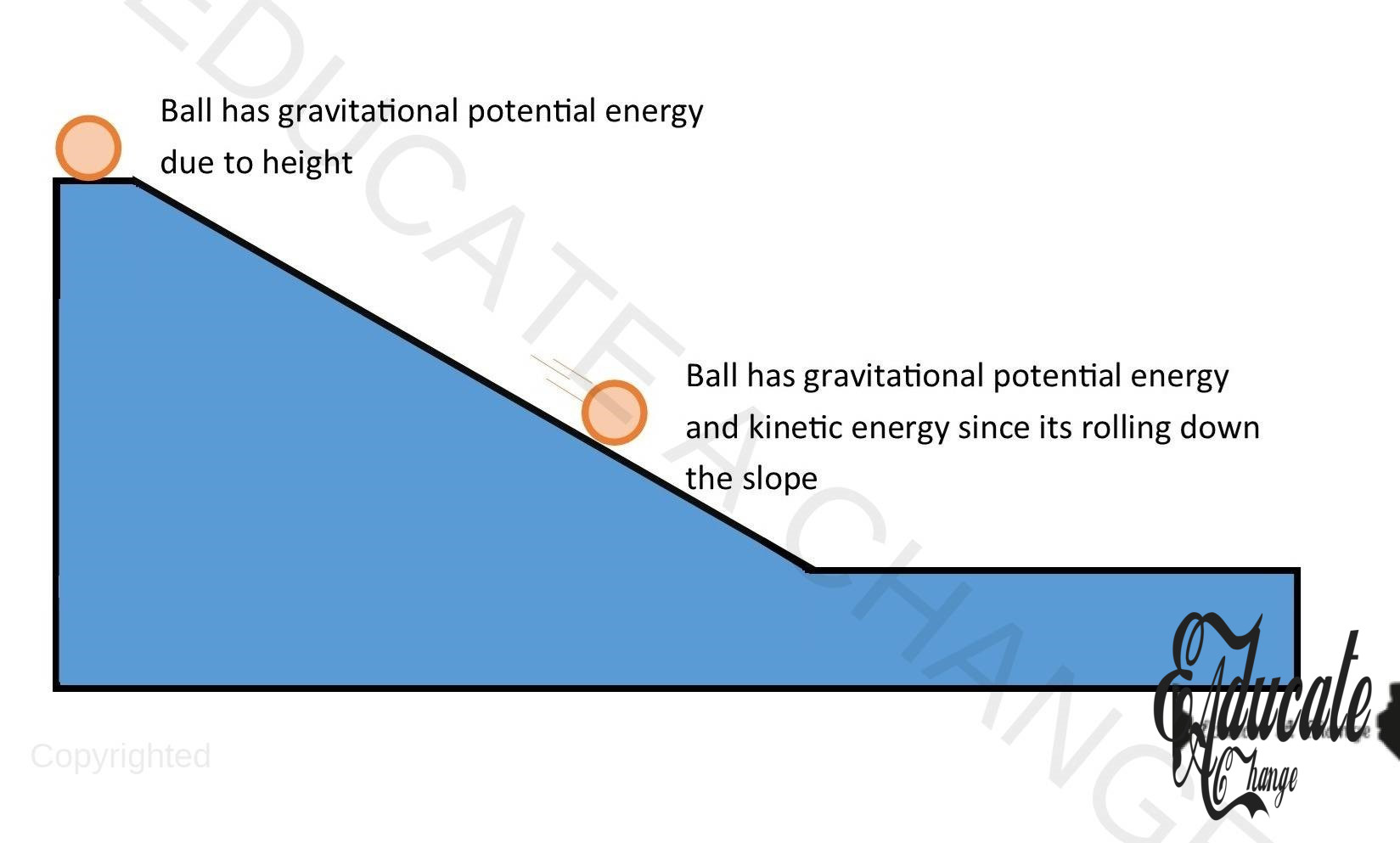
Ball Bouncing:
Consider the ball bouncing diagram:
- J-K: KE changes to GPE
- K: only GPE
- K-L: GPE changes to KE
- L:KE+ Sound+ Heat
- L-M: KE changes to GPE but less than J-K since energy is lost to surroundings
Kinetic and Potential Energy:
Kinetic Energy:
Kinetic Energy is the energy possessed by the virtue of motion of an object.
Formula:
- KE = ½ x mass x speed2
- SI UNIT: Joules
Potential Energy:
Potential Energy is the stored energy. There are three types of potential energies:
- Chemical Potential Energy
- Elastic Potential Energy
- Gravitational Potential Energy
Chemical Potential Energy:
The food we eat contains chemical potential energy that is converted to, for example, kinetic energy as we move.
Elastic Potential Energy:
Rubber bands and compressed springs contain elastic PE which is converted to KE (kinetic energy).
Gravitational Potential Energy:
- An object has gravitational potential energy (GPE) when it is raised above the Earth’s surface. When the object falls, the GPE is converted to KE.
- GPE =mass x gravitational field strength x height = mgh
- SI UNIT: joules
Work:
- Work done by a constant force is given by the product of the force and the distance moved in the direction of the force.
- Work = force x distance = Newton meters (Nm) / Joules
No work is being done:
- Direction of force being applied, and the direction of motion are perpendicular (moment)
- Force is being applied but the object does not move.
Efficiency:
- Efficiency is the percentage of useful output produced by an operation.
- Efficiency is given by
- Output will always be less than input because energy is lost in other, non-useful forms.
- For example, in fuel combustion, energy is lost in other forms such as light
Power:
Power is defined as the work done or energy converted per unit time.
Formula:
- Power = work done/time OR W/T
- SI UNIT: Watt
- One watt is the rate of energy conversion of 1 joule per unit time
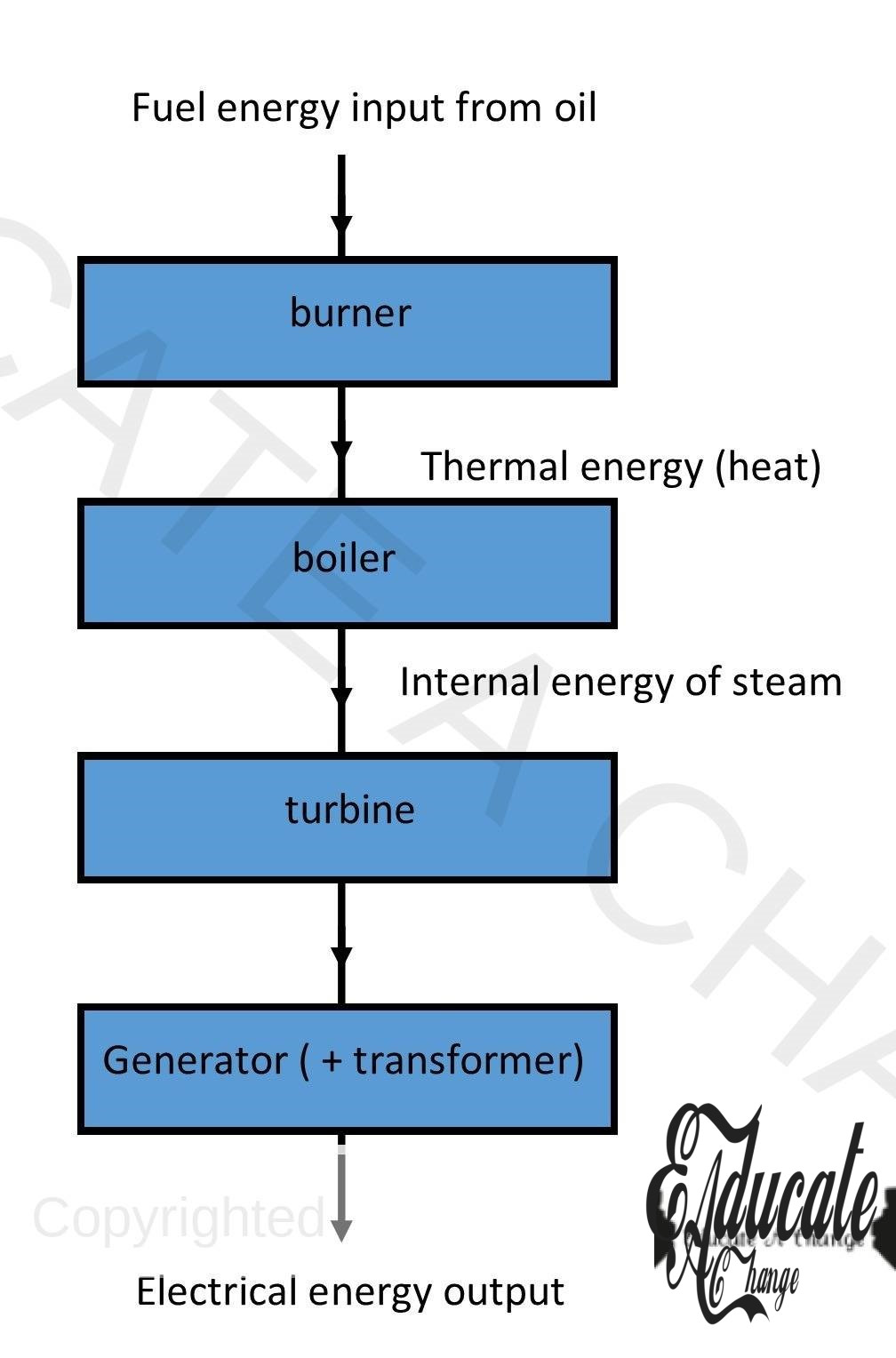
Electricity Generation Block Diagram:
Environmental Issues with Power Generation:
- Burning of non-renewable resources can wipe out supplies entirely
- Toxic gases are produced as well as unwanted ones
- Waste dumped in water can destroy marine life