Radioactivity | O Level Physics 5054 & IGCSE Physics 0625 | Detailed Free Notes To Score An A Star (A*)
Topics:
- Detection of Radiation through Electroscope
- Detection of Radiation through Cloud Chamber
- Detection of Radiation through GM Tube
- Basic Properties of Alpha, Beta and Gamma Radiation
- Ionizing Power
- Penetration Power
- Effect of electric and magnetic fields
- Radioactive Decay
- Half Life
- Decay
- Isotopes
- Nuclear Energy
- Fission Reaction
- Fusion Reaction
- Uses of Radiations
- Hazards of Radiations
- Precautions
- Background Count
- Rutherford Geiger Marsden Experiment
- Star Formation
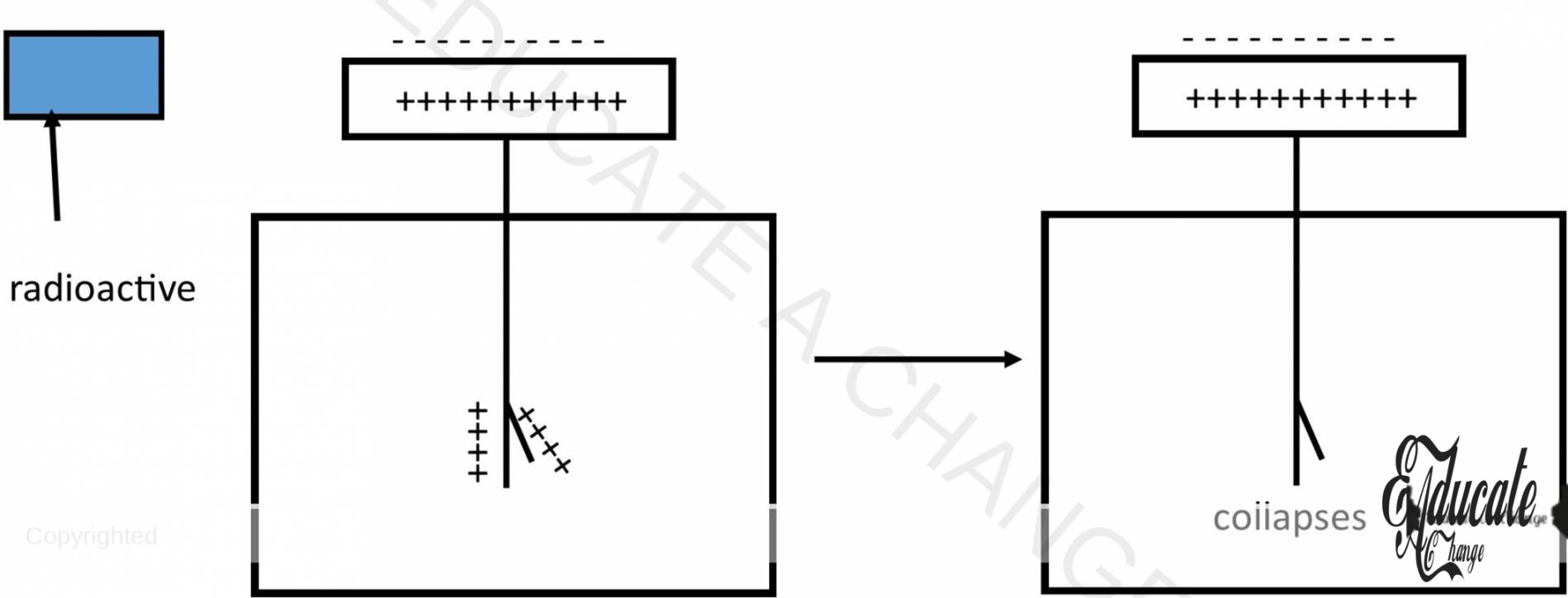
Detection of Radiations through Electroscope
- To check if there is radiation, electroscope is firstly charged,
- The gold leaf starts to show repulsion as like charges are present on both ends
- When radiations are emitted by an object, they ionize the air around the electroscope
- The opposite charged ions are attracted by the charge on the electroscope and neutralized
- This means, the charge on electroscope reduce, and gold leaf charge is removed
- Gold leaf collapses
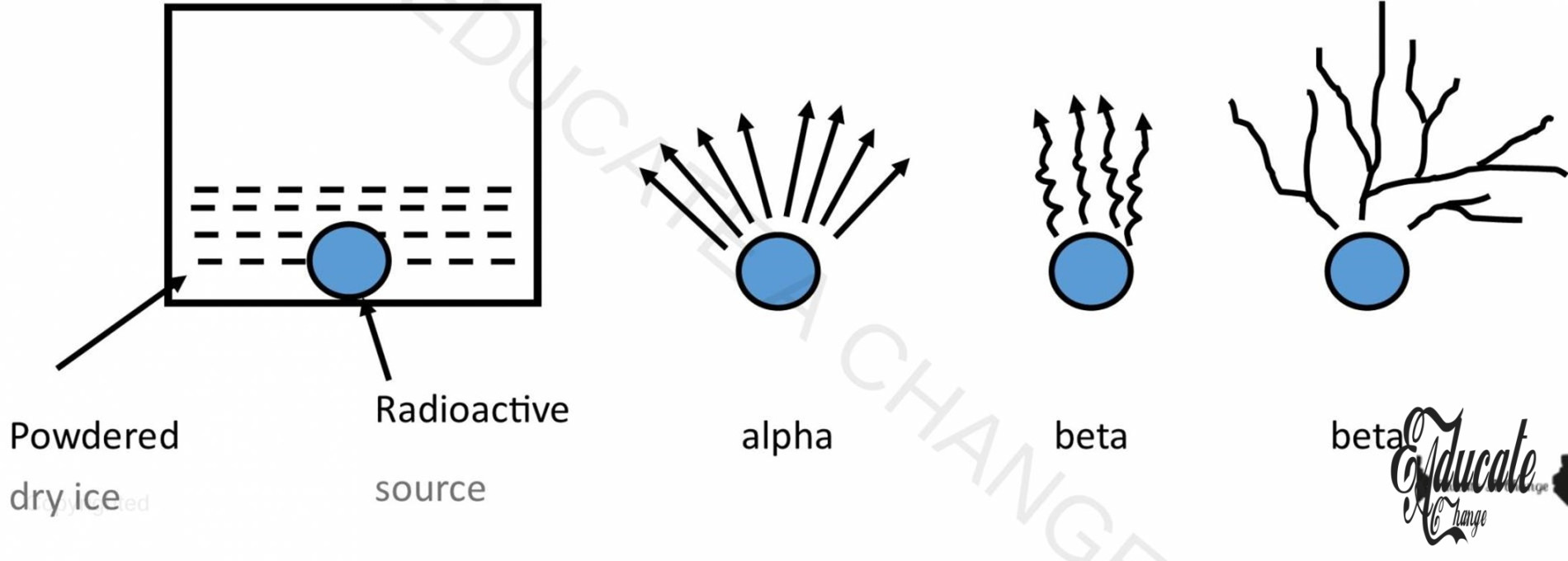
Detection of Radiations through Cloud Chamber:
- The pattern from radioactive elements are emitted that sketch a pattern on dry ice.
- Dry ice is CO2
- This pattern is checked whether its alpha, beta or gamma particles. (Pattern in diagram)
- Since Alpha is heavier than the other two, it has straight lines and no curls.
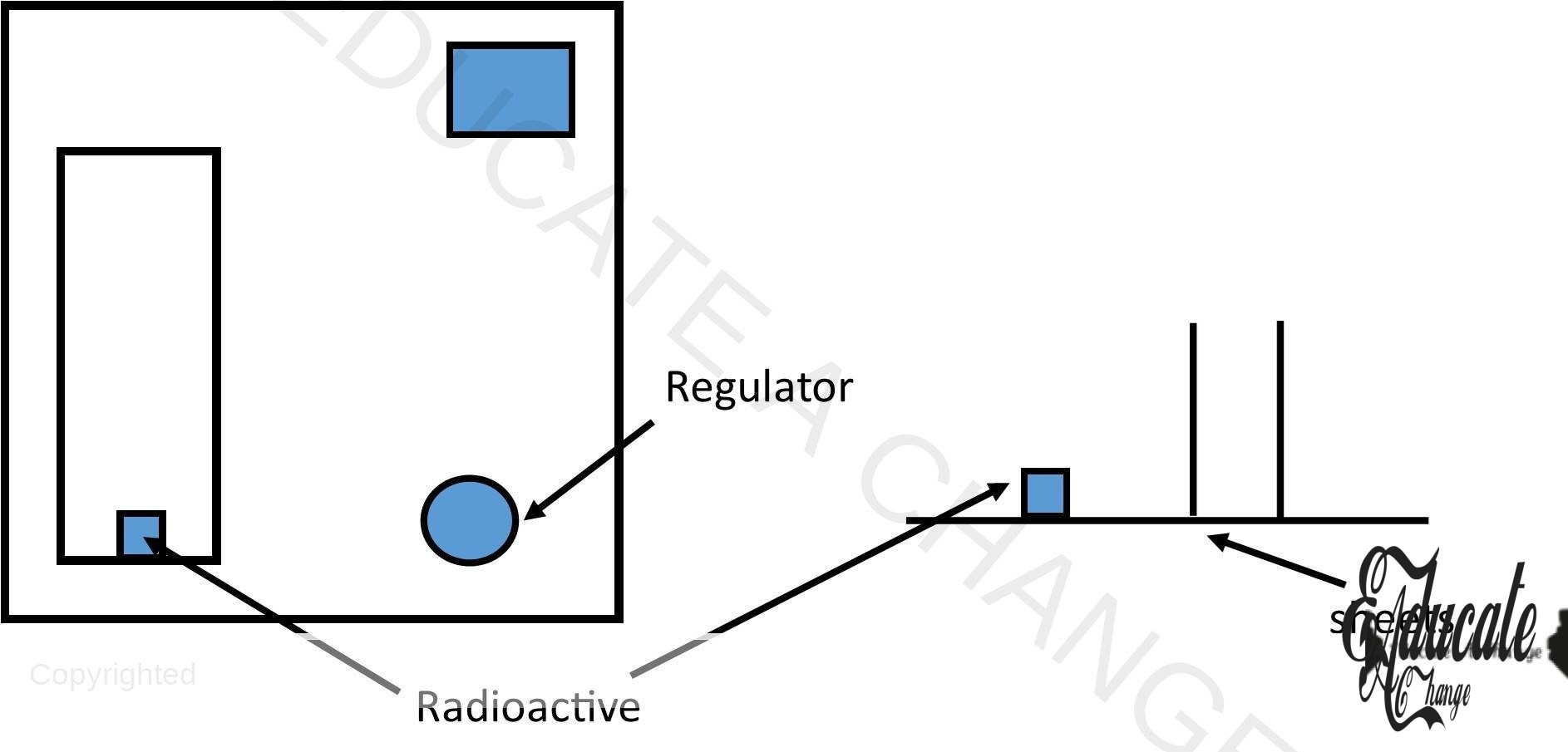
Detection of Radiations through GM Tube:
- Radioactive element is placed inside the opening of GM Tube.
- Inside, there is gas tube with argon
- The radiations go inside this tube and they ionize
- In the tube, electrodes are present that attract the opposite charges at once
- This rapid movement generates current pulse due to positive and negative ions
- The current is detected by the device and they are counted. This detects the speed and strength of the rays.
- Sheets are placed to detect whether the radiations pass by checking the count rate. (radiations per unit time)
- If the count rate drops, it means the ray is not passing a particular sheet and based on that the radiation is identified.
Basic Properties of Alpha, Beta and Gamma Radiations:
Alpha:
- ∝ or alpha rays are helium nuclei with 4 mass and 2 proton number He
- Charge = positive
Beta:
- Β or beta rays are electron. Β particle is one electron
- It has negative charge
Gamma:
- γ or gamma rays are electromagnetic waves with highest frequency
- no charge as it’s a wave
Ionizing Power:
∝ particles > β particles > γ particles
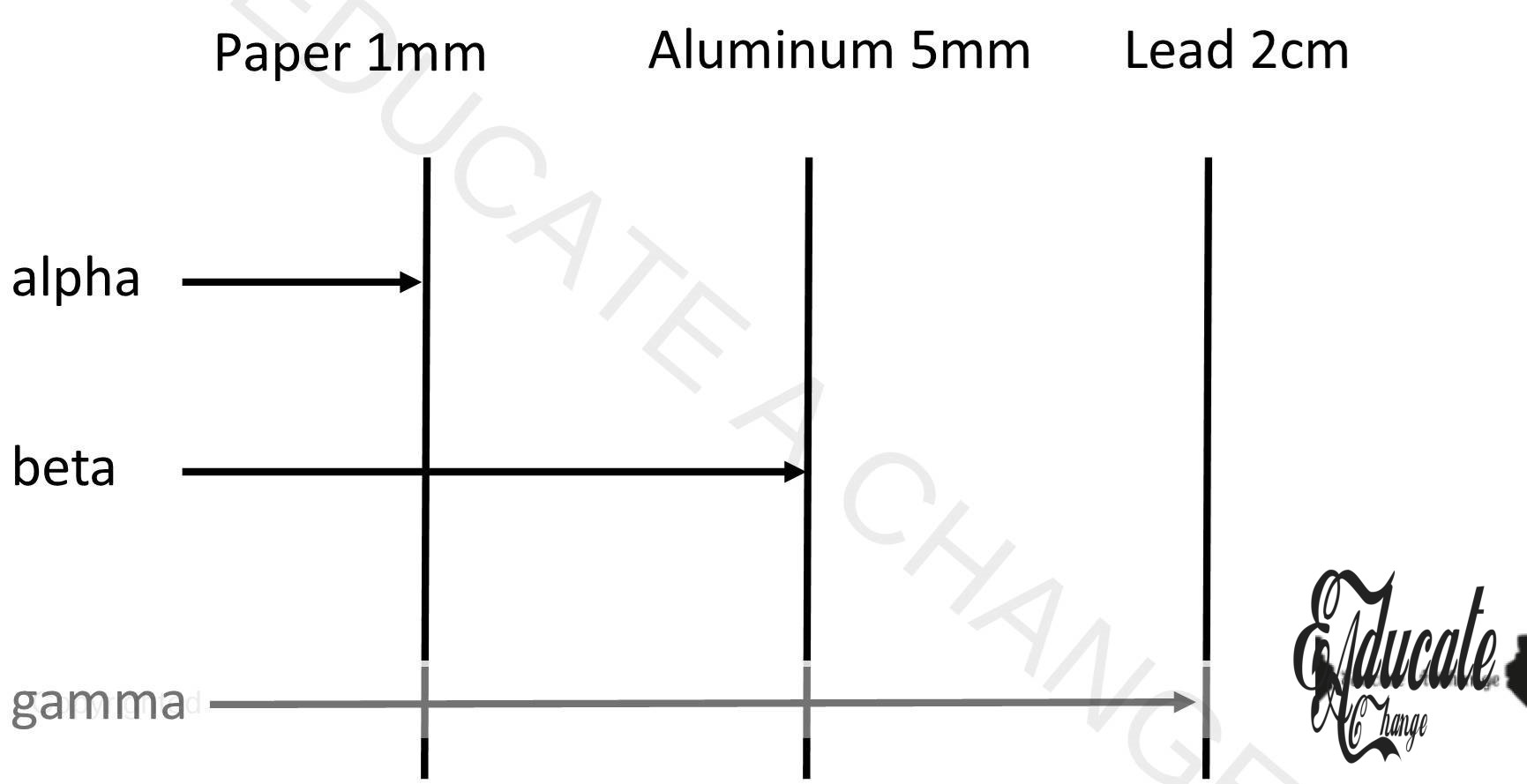
Penetration Power:
∝ particles < β particles < γ particles
- alpha cannot pass through 1mm thick sheet as they are heavy
- beta can pass paper but 5mm aluminum sheet can stop them
- gamma can pass paper and aluminum so 2cm lead is used to stop it
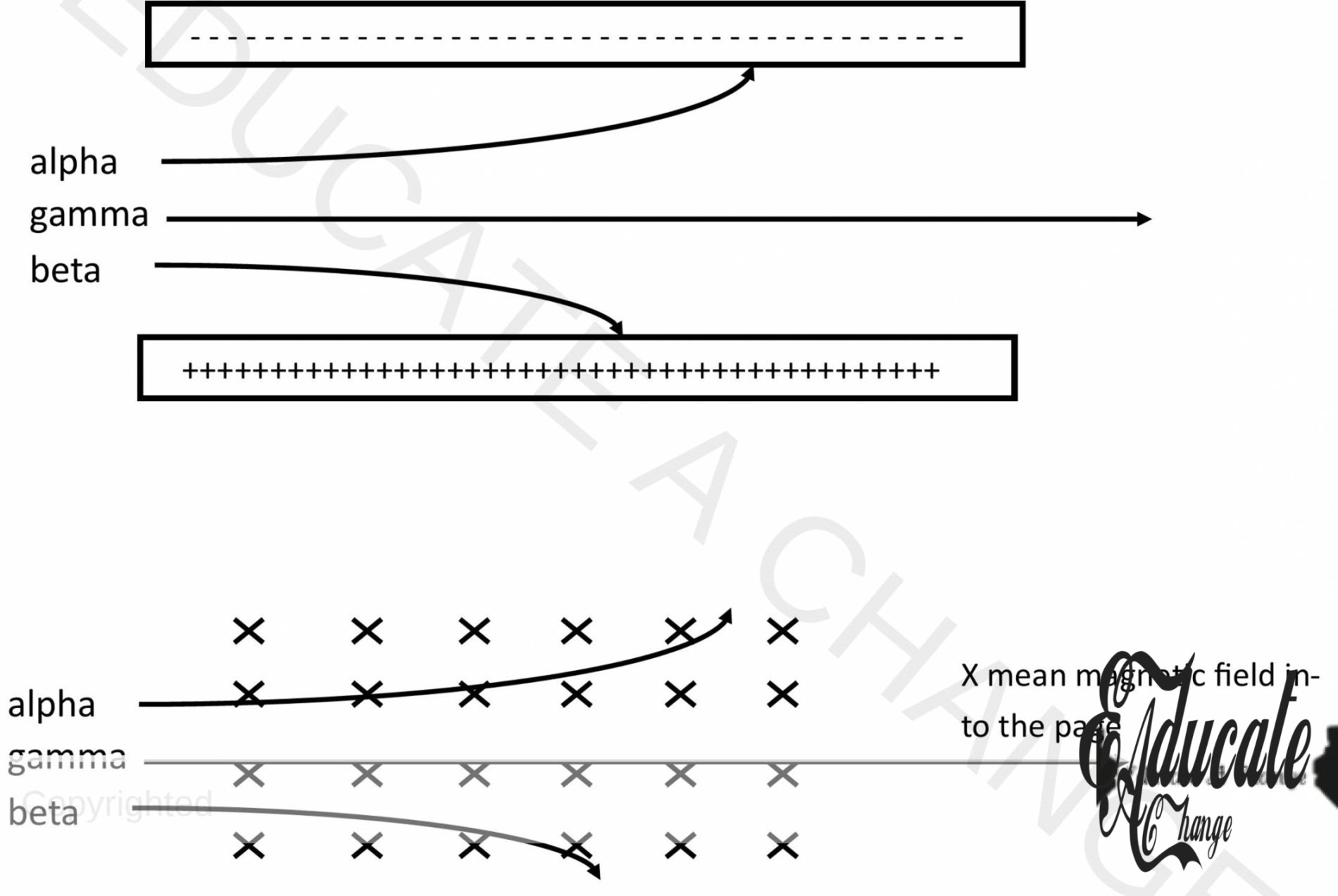
Effect of Electric Field/ Magnetic Field:
- ∝ is attracted by negative/ south
- γ is not affected
- β is attracted by positive/ north
Radioactive Decay:
- The process in which number of nuclei disintegrates to decay
- This is a random process because we cannot predict how many nuclei will disintegrate
- This process is not affected by any physical condition such as pressure, temperature etc.
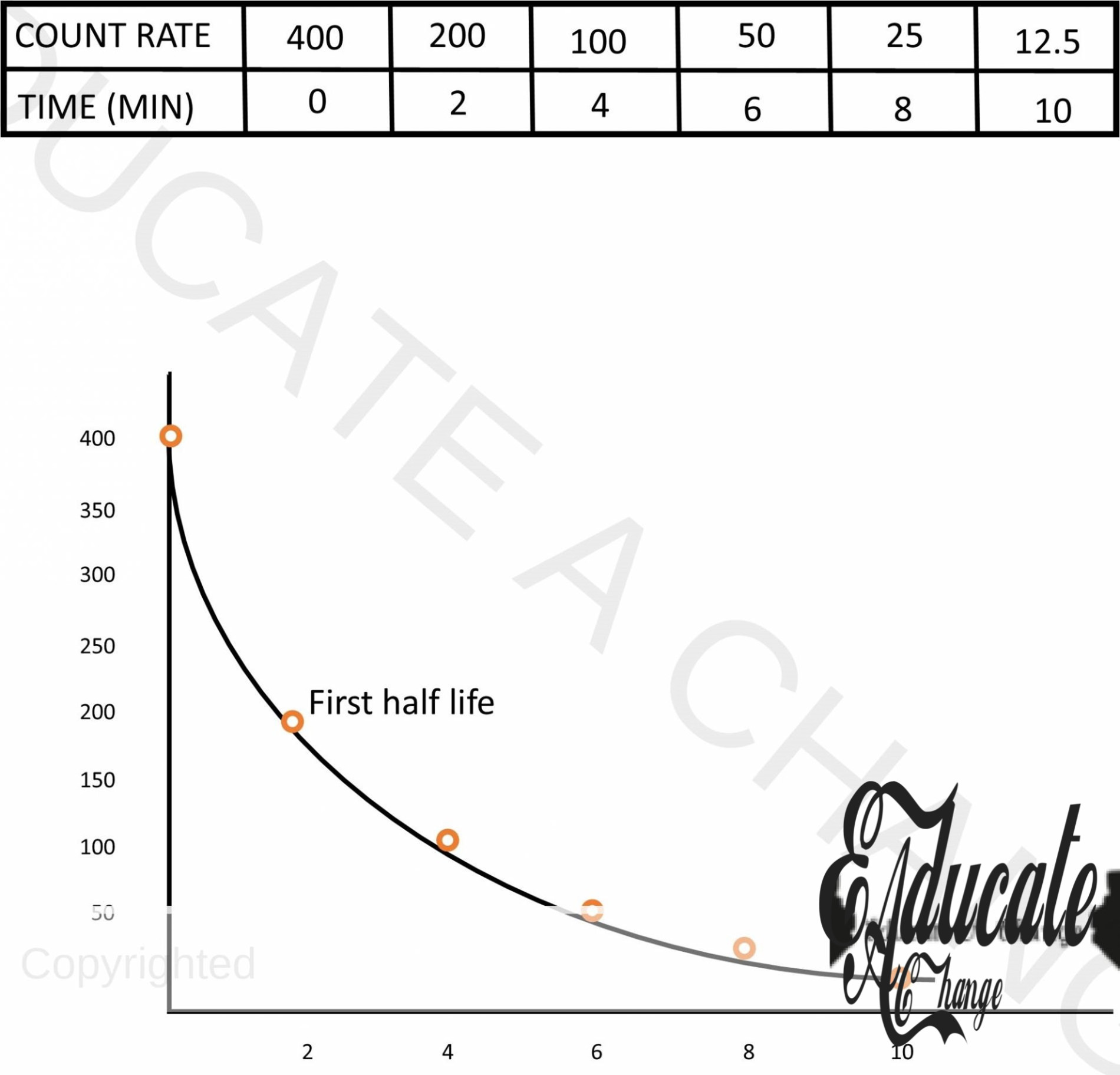
Half Life:
- The time to disintegrate half of the total nuclei is called half life
- Total time (t) = no. of half lives x half life (t1/2) (time of one only)
Decay:
Proton Number (Z):
Number of protons in the nucleus
Atomic/ Nucleon Number (A):
The total mass number including protons and neutrons
∝-decay:
- AZX → A-4Z-2X + 42∝ (as He is removed)
- Radioactive element is the parent nuclei
- The right side shows the daughter nuclei after emission of particle.
β-Decay:
- AZX → AZ+1X + e- (as electron is removed)
- As electron removes, one neutron changes into a proton but mass number remain same
γ Decay:
- NO change to nuclei
Isotopes:
Isotopes are atoms of the same element with same protons but different number of neutron and nucleon.
Nuclear Energy:
E = mc² where e is the nuclear energy, m is the mass of the substance and c is the speed of light, 3.8 x 108 m/s
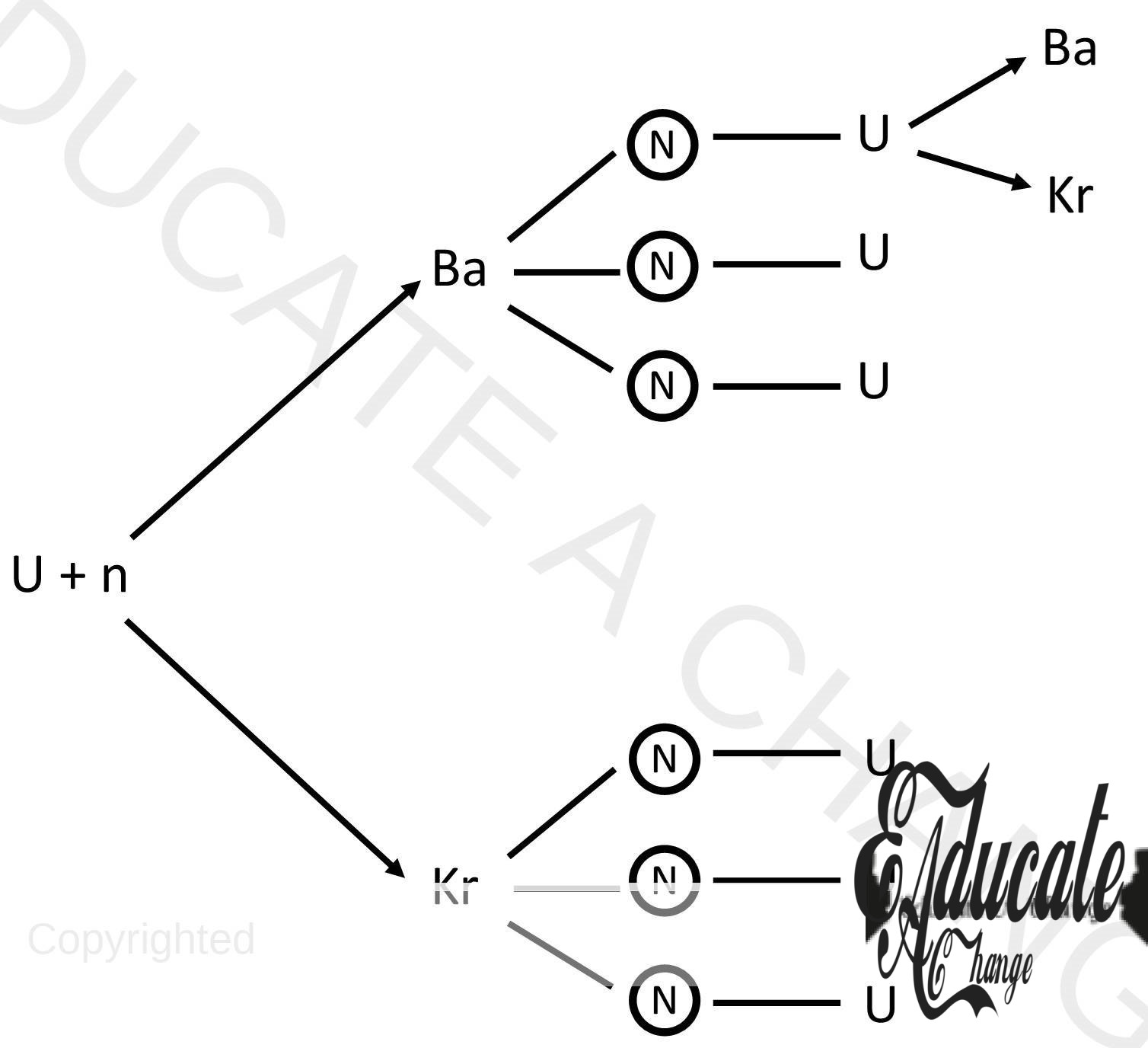
Fission Reaction:
- The process in which heavier nuclei split into smaller nuclei.
- It is a controllable reaction.
- we get new stars when heavy stars break into smaller stars.
- U + n → Kr + Ba + Energy in reactors
- Uranium reacts with neutron and becomes unstable. It breaks into two smaller elements and releases energy in the process of fission
Fusion Reaction:
- The process in which smaller nuclei join together to form bigger nuclei.
- This process is not controllable.
Uses of Radiations:
Tracers:
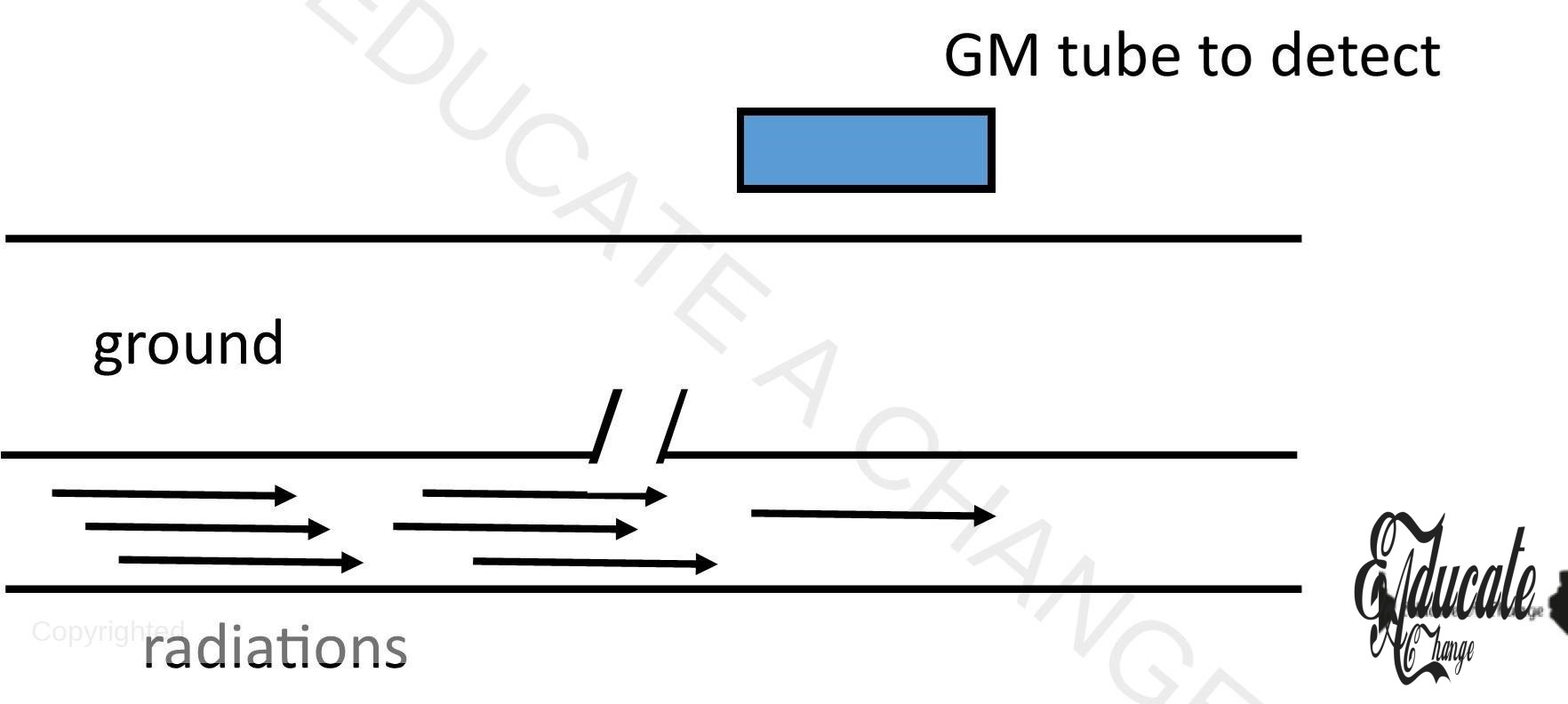
- Underground pipes.
- Radiations are involved in pipes and where they leak, they reach to the ground where they are detected by the gm tube
Medical Treatment:
Gamma rays are used to treat cancer. The cells are burned and stopped to grow forward.
Agriculture:
To make land smooth.
Archeological Dating:
Carbon dating uses radiations where we calculate the half life of carbon, and that is used to detect the age of a substance.
Hazards of Radiations:
- Cause skin cancer and other diseases
- Eyesight can be affected
Precautions:
- Use safety goggles and glasses
- Wear lead-lined suit
- Use radiation symbol where they are present for example, outside the work area
Background Count:
The count rate in the absence of any radioactive element in the GM Counter is called background count. The source of background count are outer space and cosmic rays. Also, they can be caused by underground radioactive rocks. Remaining amount = original amount x (1/2)t — number of half lives
Rutherford Geiger Marsden Experiment:
In the alpha scattering experiment, alpha particles were passes in a gold foil. Most of the particles went through and only small quantity was reflected back. This meant that the nucleus that repels the alpha particle is very small as compared to the entire atom. In simpler words, most areas were empty and the heavy nucleus was very small.
Star Formation:
- Massive clouds of dust and gas form stars.
- Gravity pulls stars and dust together and as it gets hotter, nuclear reaction starts.
- This also produced energy which keeps the star hot and keep the reaction going.