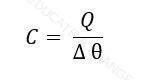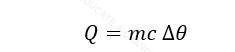Thermal Properties of Matter and Kinetic Model of Matter | O Level Physics 5054 & IGCSE Physics 0625 | Detailed Free Notes To Score An A Star (A*)
Topics:
- Kinetic Model of Matter
- States of Matter
- Kinetic Molecular Model of Matter
- Brownian Motion
- Gas Laws
- Thermal properties of matter
- Heat Capacity and Specific Heat Capacity
- Melting and Freezing
- Boiling and Condensation
- Specific Latent Heat
- Boiling and Evaporation
- Factors affecting state changes
Kinetic Model of Matter:
States of Matter:
Matter:
Any substance that occupies some space and has some mass is called Matter.
States of Matter:
States of Matter mean the forms in which matter exists in our environment. There are three states of Matter:
- Solids
- Liquids
- Gases
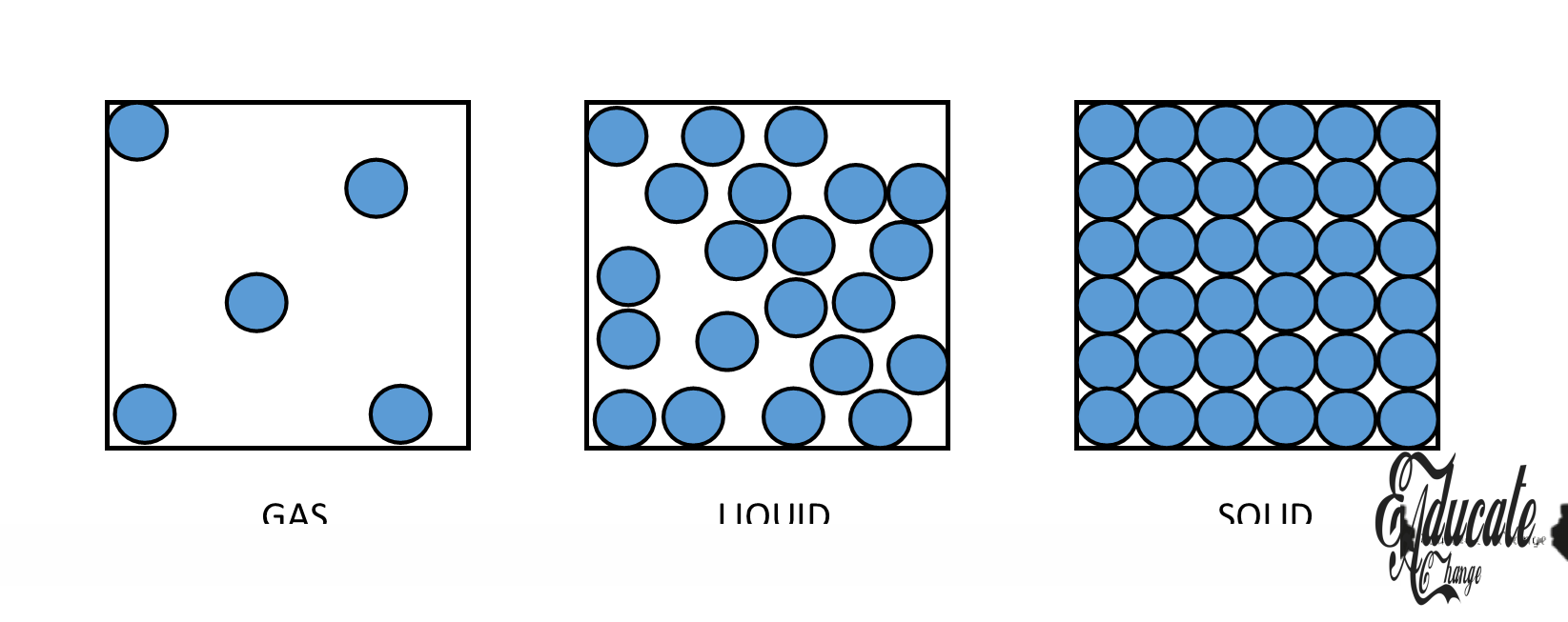
Properties of States of Matter:
The table below tells us about some of the important properties of the three states of matter.
| Properties | Solids | Liquids | Gases |
| Shape | Fixed shape | Takes container’s shape | Takes container’s shape |
| Volume | Fixed volume | Fixed volume | Takes container’s volume |
| Compressibility | Cannot be compressed | Very hard to compress | easily compressed |
| Particle arrangement | Tightly packed | Loosely packed | Far apart |
| Intermolecular forces | Very strong | Weak | Very weak |
Kinetic Molecular Model of Matter:
We know that matter is made up of atoms and molecules and they are in continuous random motion, this is called Kinetic Molecular model of matter. Let us look at the arrangement and movement of particles in the three states of matter:
| States | Arrangement of particles | Movement of particles |
| Solids | · Closely packed together in regular shape, · Rows of particles, · Results in high density | · Vibrates at a fixed position, · Strong intermolecular bond |
| Liquids | · Loosely and randomly packed together in shape of container, · Relatively high density | · Move randomly in all directions, · Relatively strong intermolecular bonds |
| Gases | · Freely packed, · Move in any available direction in shape of container, · Relatively low density | · Move in all directions randomly, · Weak intermolecular bonds. |
Brownian Motion:
Objectives:
To study random motion of particles or Brownian motion
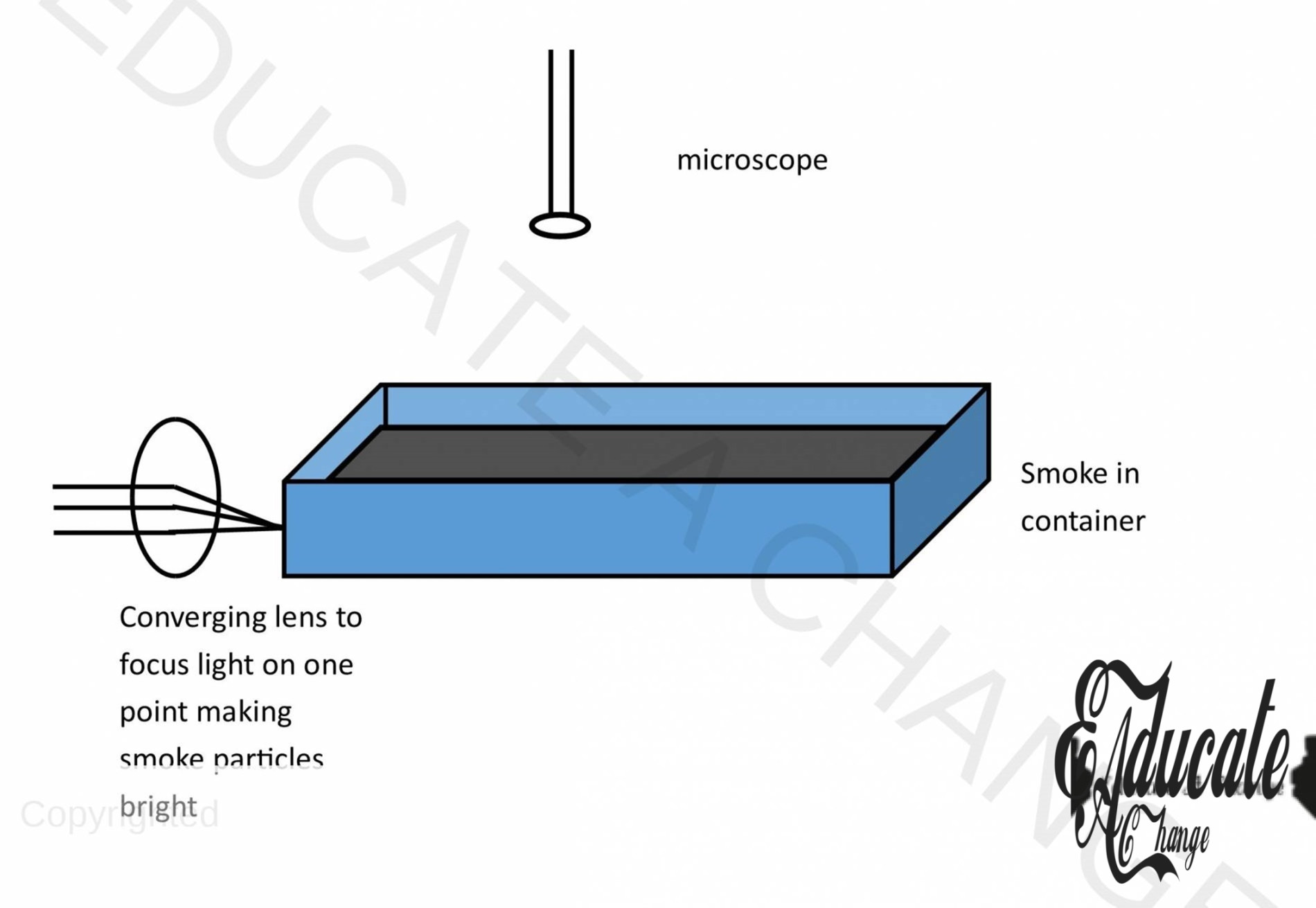
Apparatus:
Torch light, glass cell containing smoke, microscope
Procedure:
- Assemble the apparatus as shown in the diagram,
- Seal a glass cell containing smoke and place a microscope above it
- Observe the motion of the smoke particles.
Observation:
- The smoke particles scatter the light shining on them, so that they can be seen
- The smoke particles are seen moving in irregular patterns.
- Larger the particles are, less haphazard the motion is,
Conclusion:
- When air particles strike on the smoke particles, the particles move in irregular patterns,
- When particles are large, the striking of air particles would have less effect as air particles are small so movement would be less.
Gas Laws:
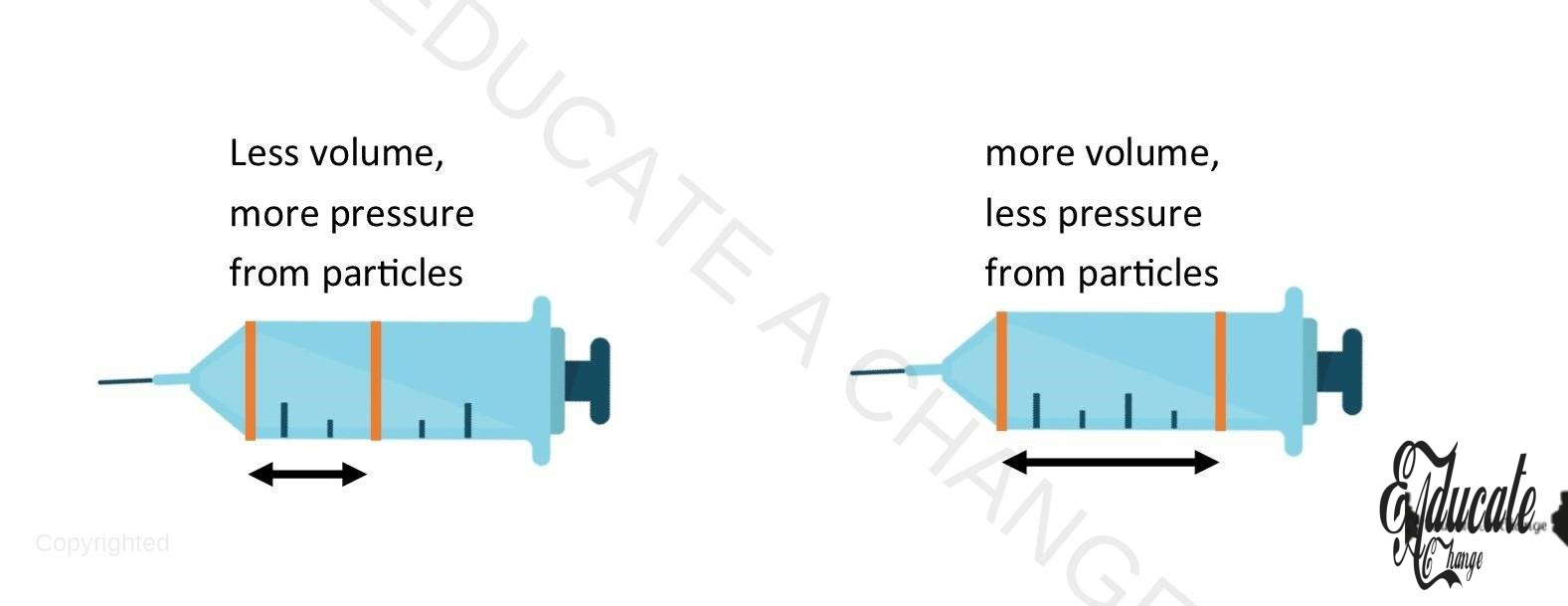
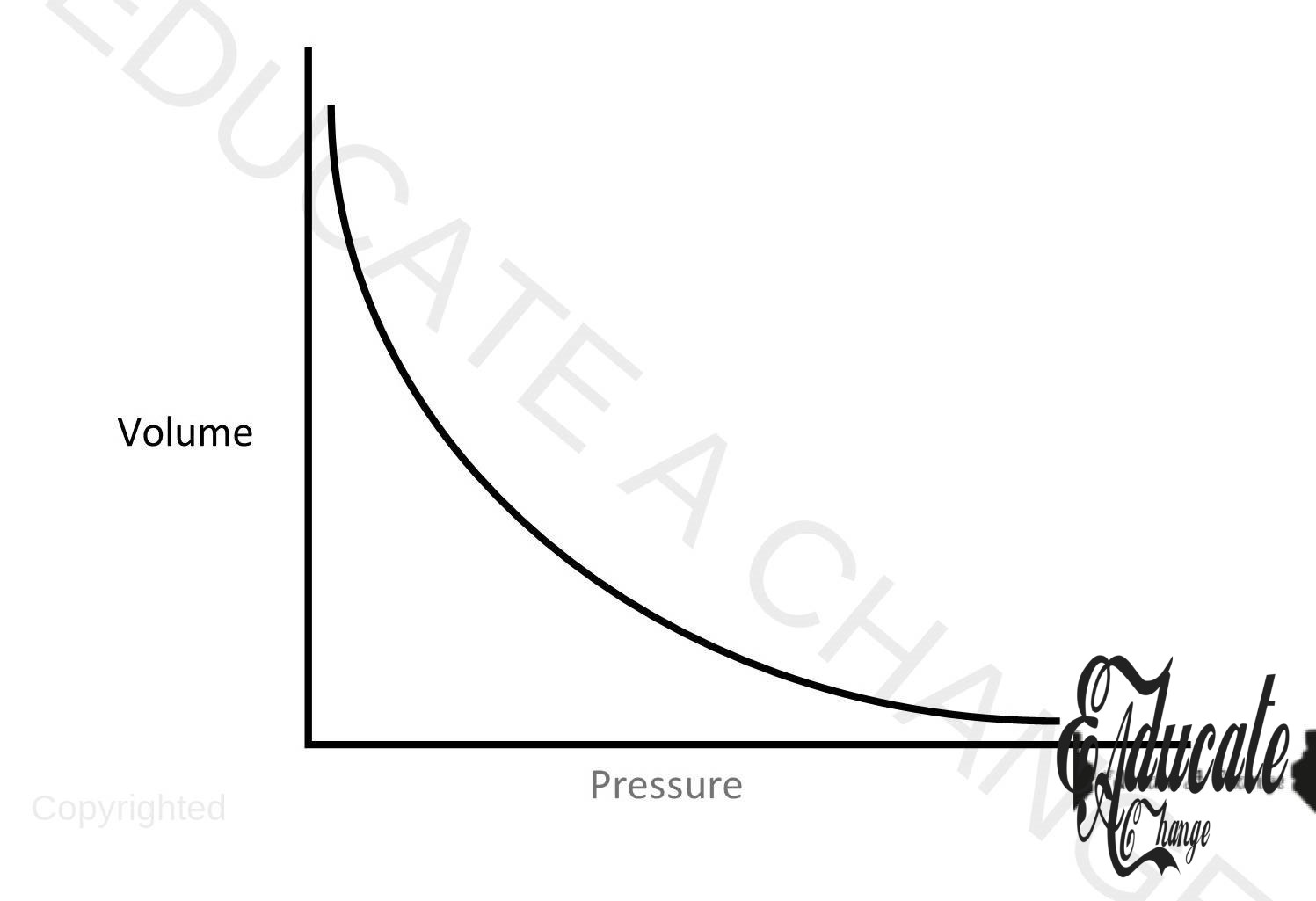
Boyle’s Law:
- The volume of given mass of gas is inversely proportional to pressure at constant temperature.
- Volume ∝ 1/Pressure
- Volume = K / pressure
- Volume x pressure = k
- VP = K
- P1V1 = K; P2V2=K
- P1V1 = P2V2
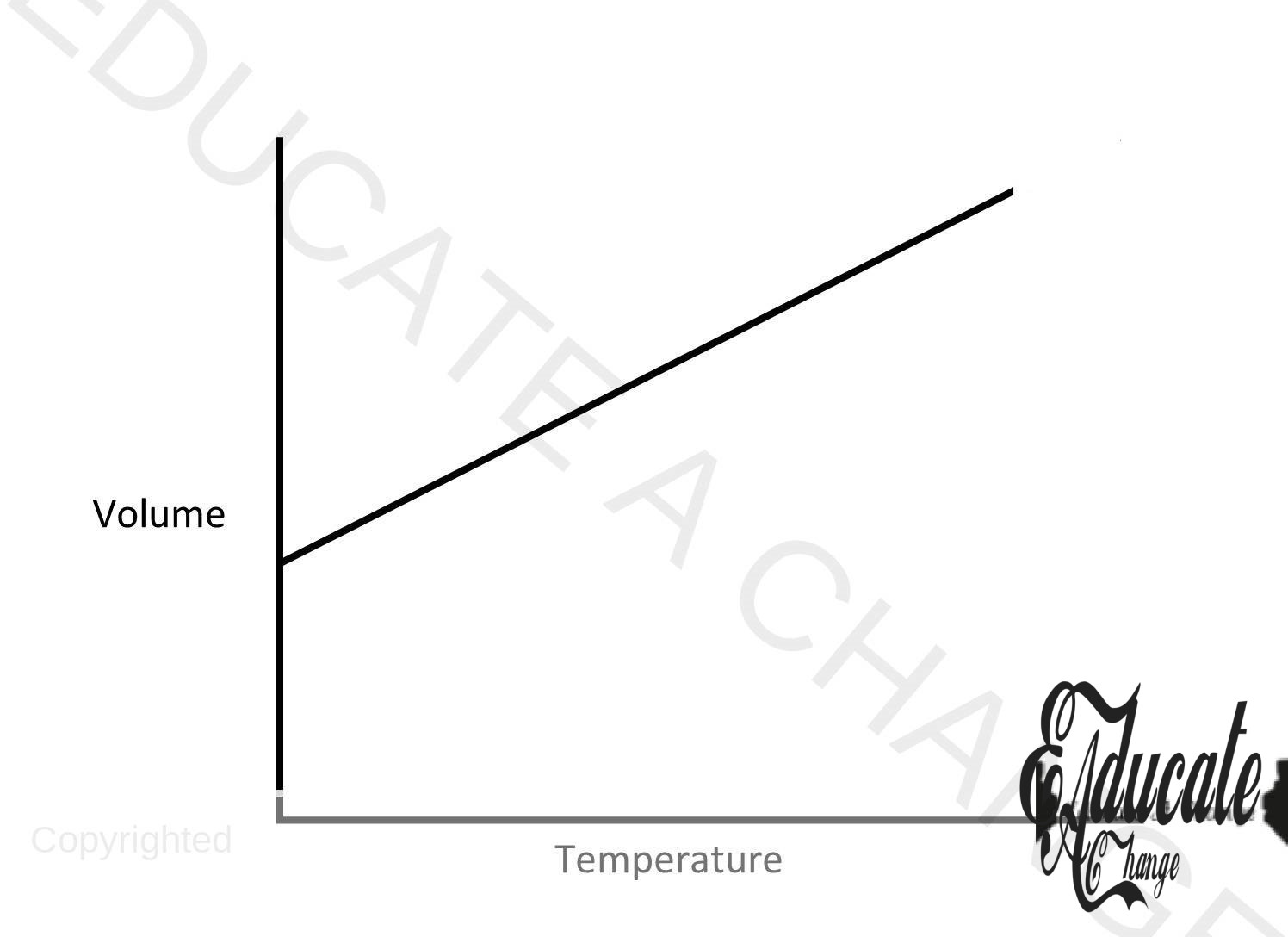
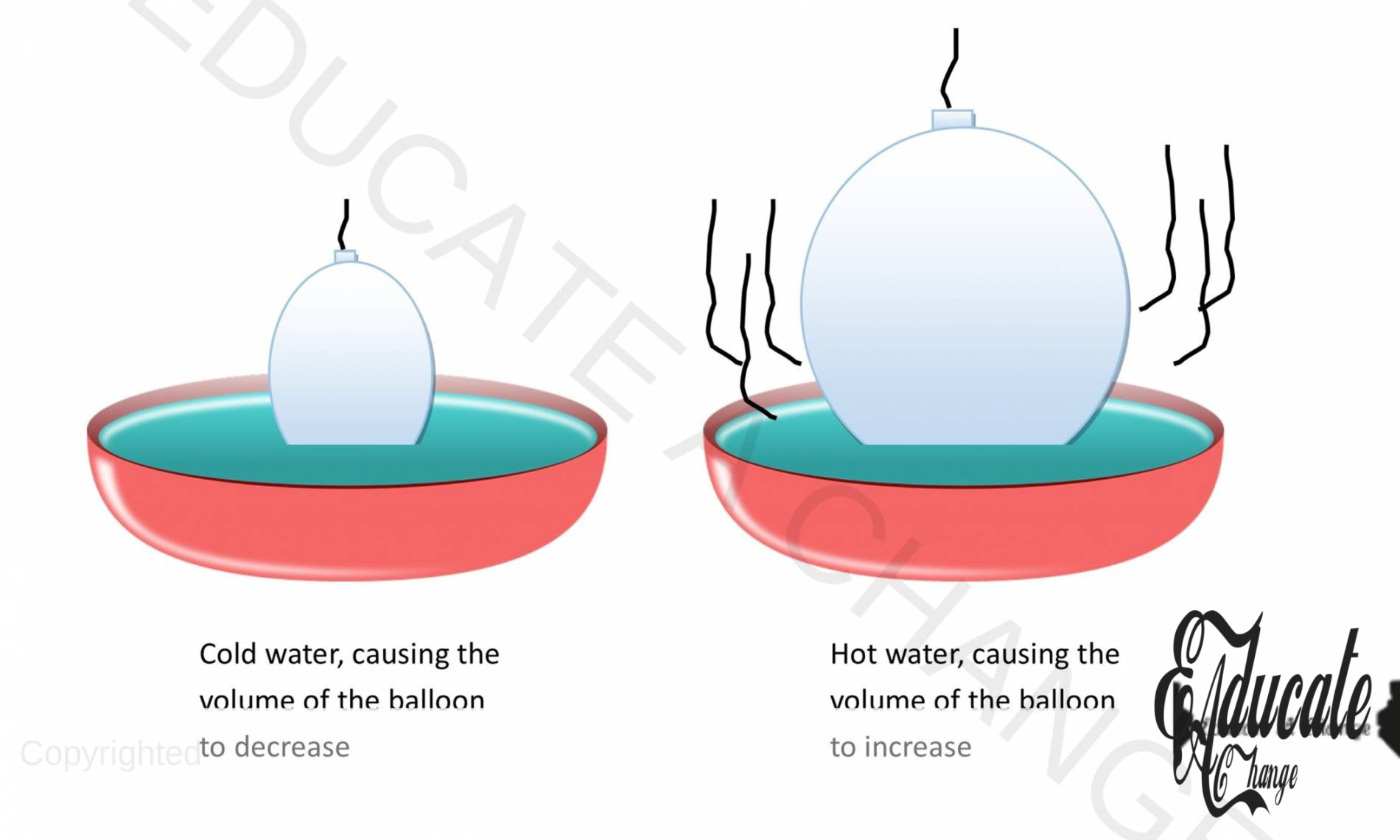
Charles Law:
- The volume f a given mass of gas is directly proportional to temperature at constant pressure.
- Volume ∝ temperature
- Volume = K (temperature
- V/T = K
- V1/T1 = K; V2/T2=K
- V1/T1= V2/T2
Thermal properties of matter:
Heat Capacity and Specific Heat Capacity:
Heat Capacity:
The amount of heat required to raise the temperature through 1K (kelvin) of a substance.
Where
- C = heat capacity, (J/K)
- Q = Heat Energy, (J)
- ∆θ = Change in temperature, (K)
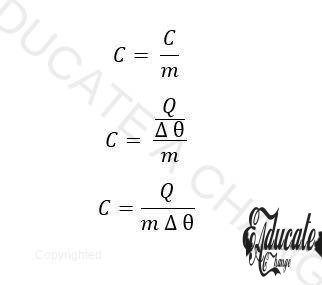
Specific Heat Capacity:
The amount of heat required to raise the temperature through 1K of a 1kg substance.
Where m = mass (kg) and c = specific heat capacity (SI UNIT: J/kg K)
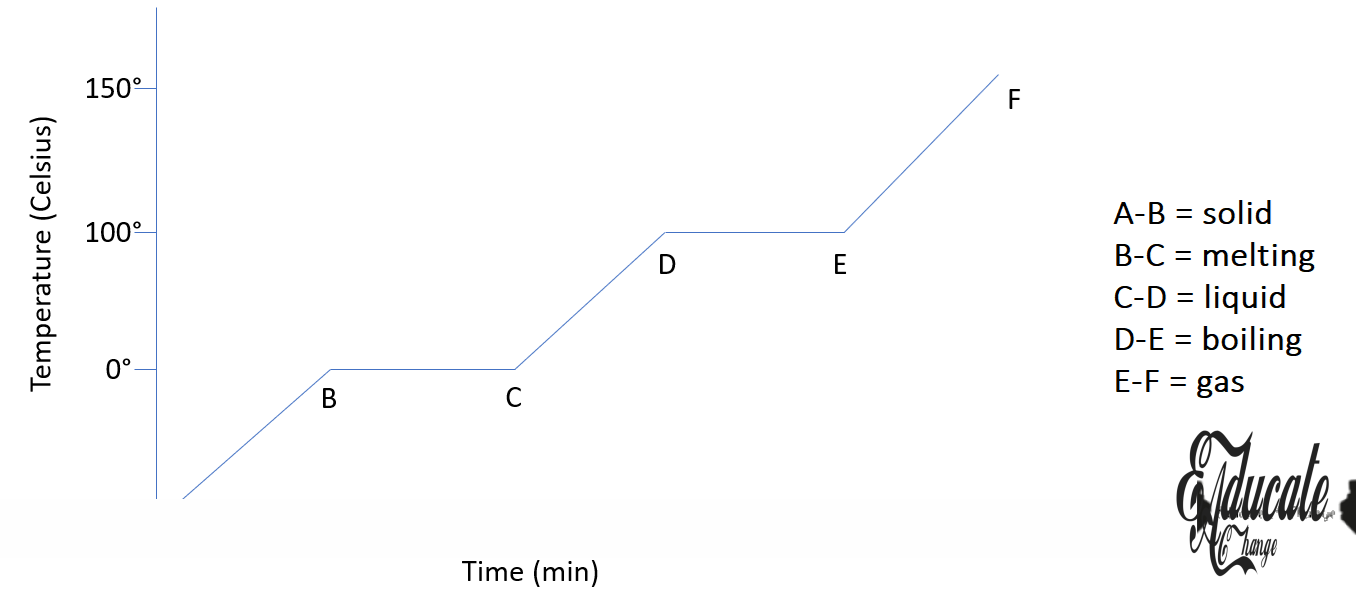
Melting and Freezing:
Melting Point:
The temperature at which solid changes to liquid.
Freezing Point:
The temperature at which a liquid change to solid.
Latent Heat of Fusion:
Amount of heat required to change the state of matter from solid to liquid without changing temperature.
Boiling and Condensation
Boiling Point:
Temperature at which liquid starts changing to vapors.
Condensation Point:
The temperature at which vapor/gas starts changing to liquids.
Latent Heat of Vaporization:
Amount of heat required to change the state of matter from liquid to gas without changing temperature.
Specific Latent Heat:
Specific Latent Heat of Fusion:
Amount of heat required to change the state of matter of 1kg substance from solid to liquid without changing temperature.
- Lf = lf x mass
- Where:
- lf = specific latent heat of fusion,
- Lf = latent heat of fusion,
- m = mass
Specific Latent Heat of Vaporization:
Amount of heat required to change the state of matter of 1kg substance from liquid to gas without changing temperature.
- Lv = lv x mass
- Where:
- Lv = specific latent heat of vaporization,
- Lv = latent heat of vaporization,
- m = mass
Boiling and Evaporation:
| Evaporation | Boiling |
| Turning of liquid into a gas at a temperature lower than boiling point | Turning of liquid into a gas at a constant temperature called boiling point. At a temperature higher than this point, the substance cannot exist in a liquid state. |
| Slower process | Much faster process |
| Occurs on the surface of the liquid | Occurs throughout the liquid |
Factors affecting state changes:
Factors affecting freezing point:
Adding impurities lower the freezing point.
Factors affecting melting point:
Increasing the pressure lowers melting point.
Factors affecting boiling point:
Increase in atmospheric pressure will increase boiling point.
Factors affecting evaporation:
- temperature,
- surface area,
- humidity,
- surrounding air.