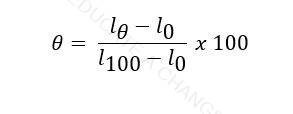Transfer of Thermal Energy | O Level Physics 5054 & IGCSE Physics 0625 | Detailed Free Notes To Score An A Star (A*)
Transfer of Thermal Energy – Temperature
Topics:
- Transfer of Thermal Energy
- Conduction
- Convection
- Radiation
- Total Transfer
- Temperature
- Heat and Temperature
- Measuring Temperature
- Liquid-In-Glass thermometers
- Thermocouple
- Range, Sensitivity and Responsiveness
Transfer of Thermal Energy
Conduction
Definition:
Conduction is the process in which thermal energy is passed through without transfer of the material itself.
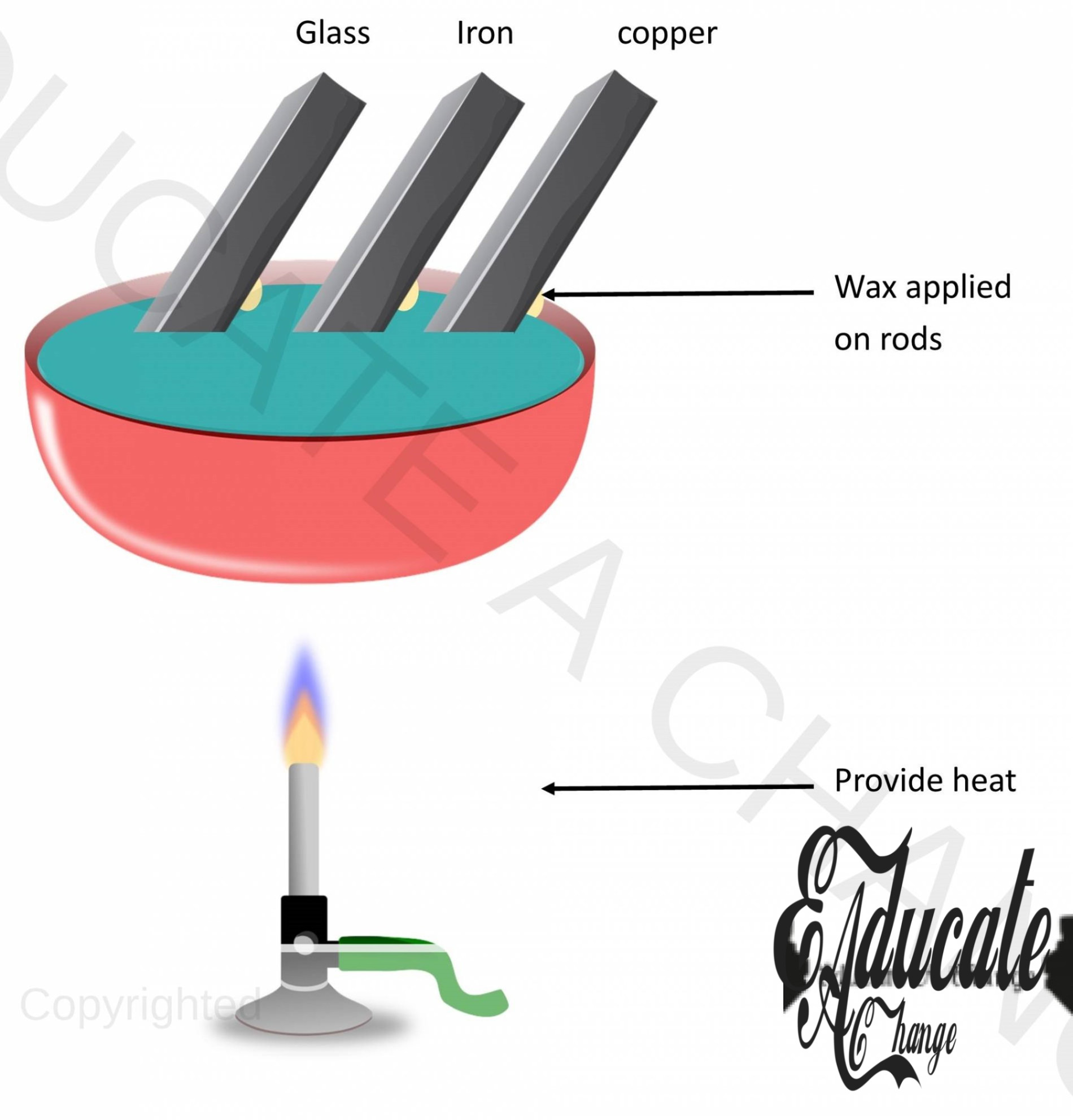
Experiments in solid:
Objective: To observe the conduction process. Apparatus: Bath, rods of same dimensions but different material, wax, stopwatch. Procedure:
- Put the rods coated with wax in the tub,
- Pour hot water in the bath and observe the timing for the melting of the wax n the rods,
- The wax will melt at different time intervals for different materials.
Conclusion:
- Heat transfers without transferring of medium itself,
- Rate of heat transfer is different on all materials,
- Heat is transferred through the collision of atoms and molecules. Metals have free electrons to transfer thermal energy.
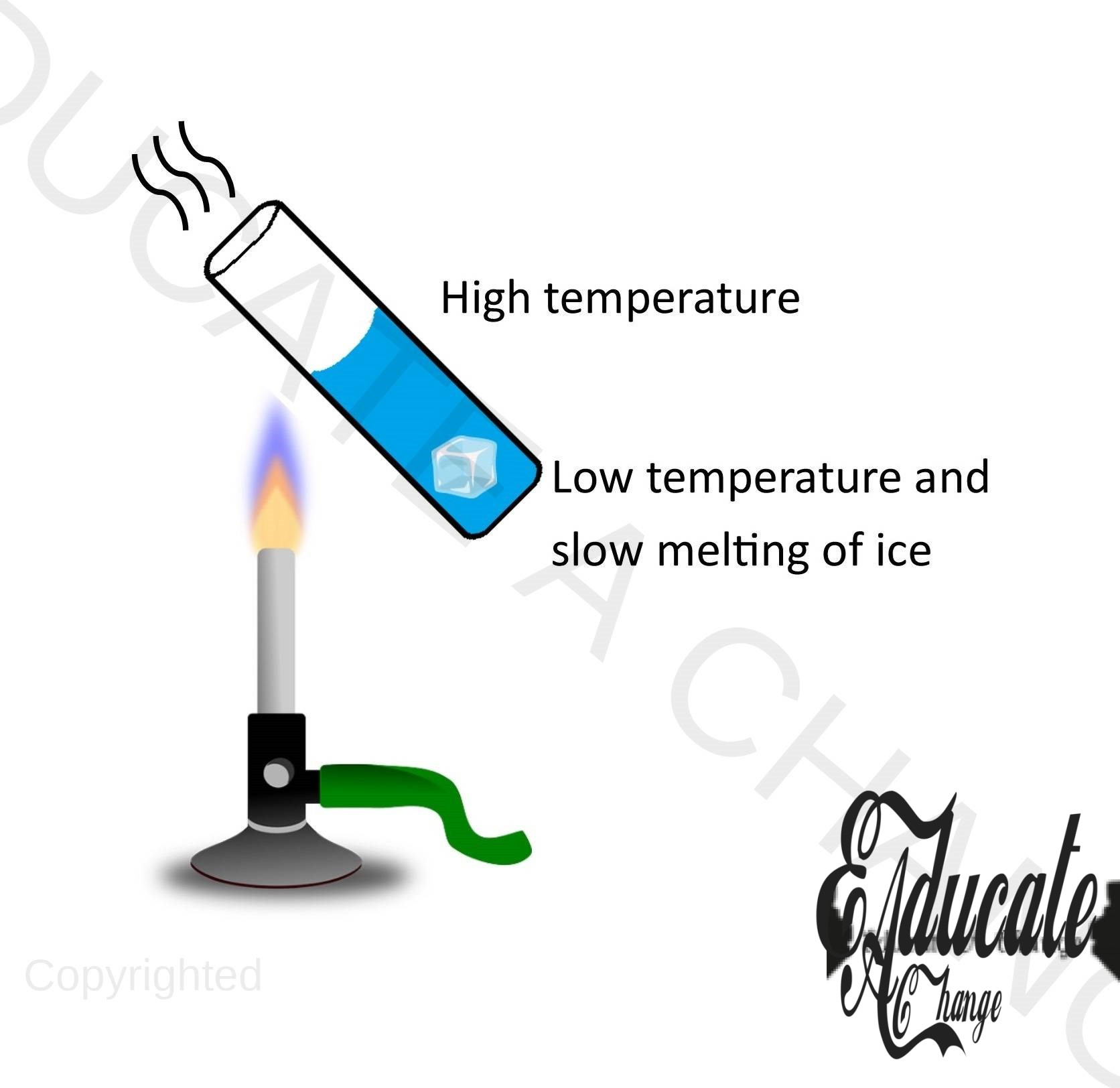
Experiment in liquid:
Objective: To observe conduction in liquids. Apparatus: Test tubes, metal covered with ice, burner, water. Procedure:
- Place the test tube over the burner in a tilted way so that the upper half of the test tube receives heat,
- Observe that the water on the upper surface will heat up but the ice will melt slowly,
Conclusion:
- Rate of transfer if energy is slow in water as water is a poor conductor of heat.
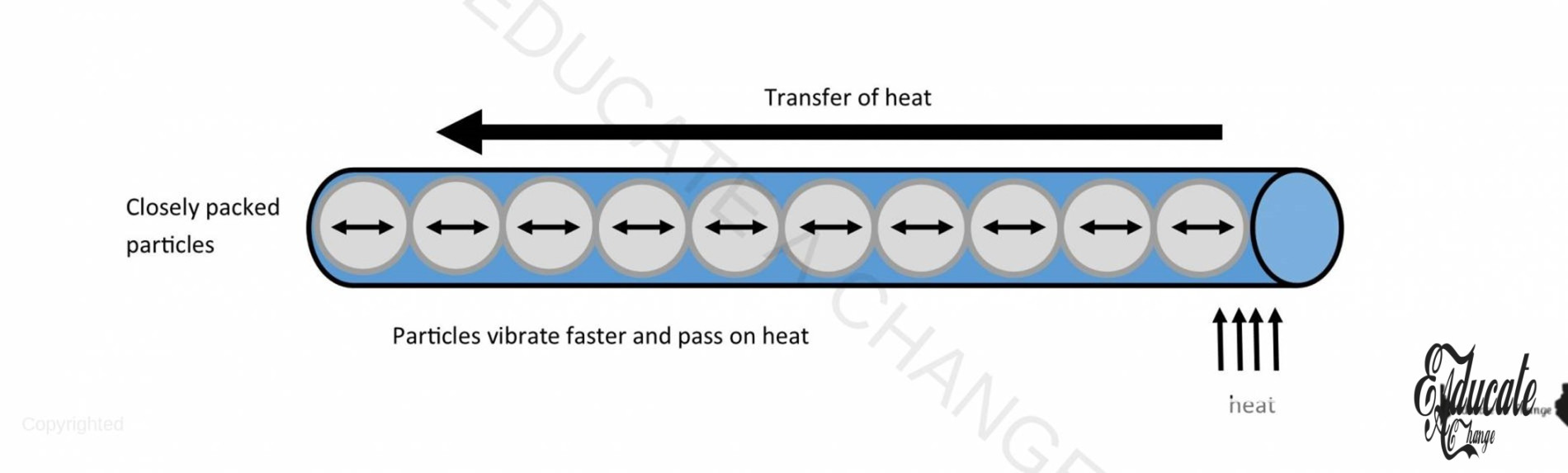
Conduction in Solids:
- When a solid is heated, the particles gain energy and they start to vibrate faster. As they vibrate, they collide with the neighboring particles and transfer the heat to those particles.
- In metallic solids, there are free moving electrons which transfer the heat from one end to the other end.
- The rod physically does not move.
Convection:
Definition:
Convection is the transfer of thermal heat energy through currents in fluids (water and gases).
Experiment in liquids:
Objective: To observe convectional current in water, Apparatus: Round bottom flask, potassium permanganate, Bunsen burner, water, Procedure:
- Heat the water in the flask having potassium permanganate,
- Purple streams can be seen rising up and sinking down as color of potassium permanganate is released and moves with the currents,
- Hot water rises and cold takes its place. This cycle continues and all the water becomes hot and purple.
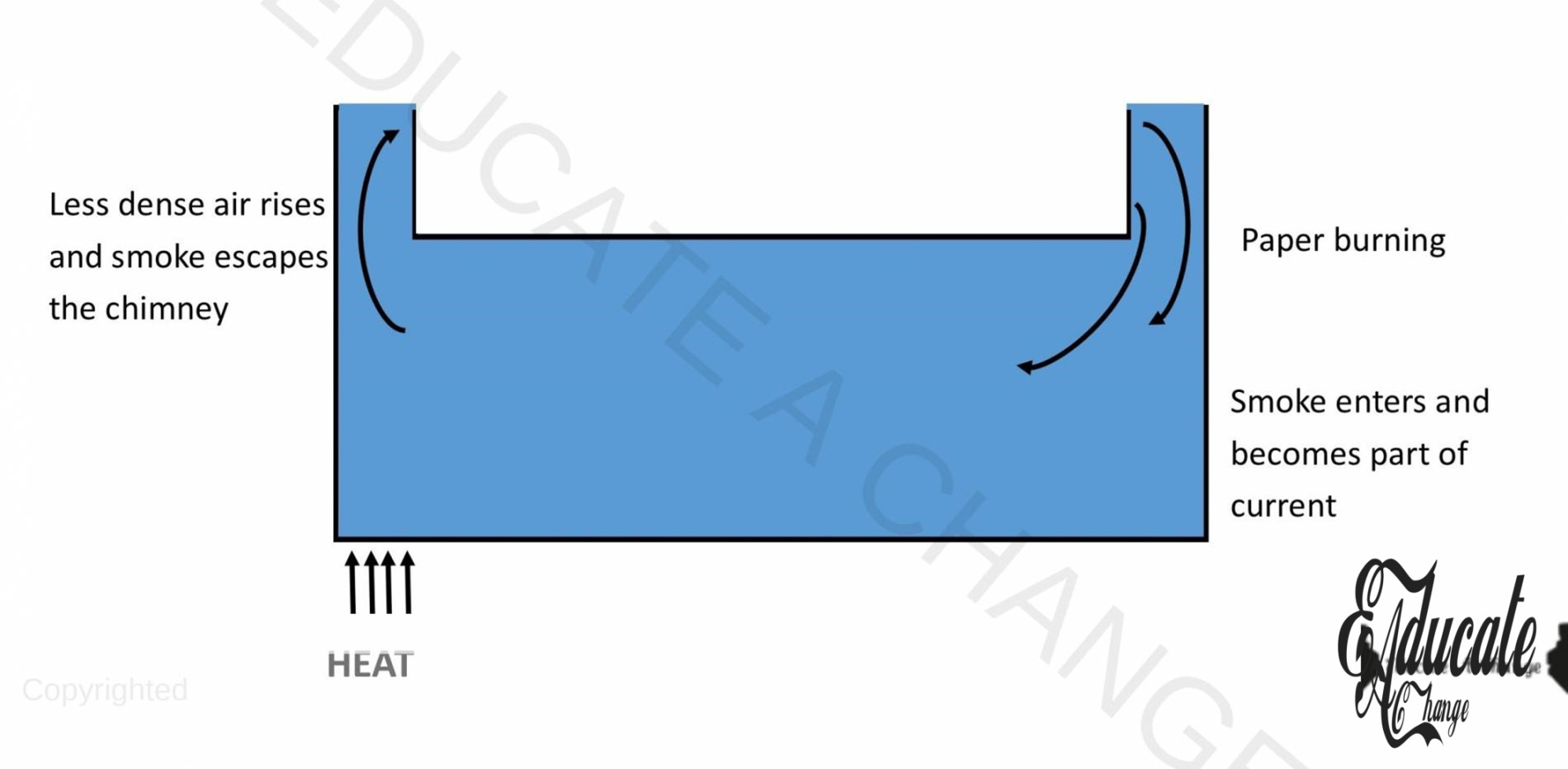
Experiment in Air:
Objective: To observe convectional current in air, Apparatus: Large box with two chimneys, paper, candle, matchbox, Procedure:
- Burning candle is placed below one chimney and paper is burned on the other side.
- The smoke of burning paper enters the box from one chimney and it finds it’s passage out of the other chimney as candle heats up surrounding air and it becomes less dense and rises up.
Conclusion of Experiments:
- Water in round bottom flask, once heated rises up, expands and gets less dense. The denser layer sinks down and takes its place.
- Similarly, in convection in air, the air that is in contact with the burning candle expands, becomes less dense and rises up. The denser air will sink and fill the space.
Radiation:
Definition:
- Radiation is a continuous emission of infrared rays from the surface of all the bodies, without the aid of a medium.
- Radiation does not require a medium for energy transfer so it can pass through vacuum.
Experiment for absorbing infrared rays:
Objective: To investigate the absorption of infrared rays. Apparatus: Two temperature sensors, dataloggers, aluminum foil, black felt pen, bulb. Procedure:
- Color one piece of the aluminum foil black using the pen.
- Wrap two aluminum foils, shiny and black on the temperature sensors,
- Set the data logger with the temperature sensors,
- Use the bulb to provide rays,
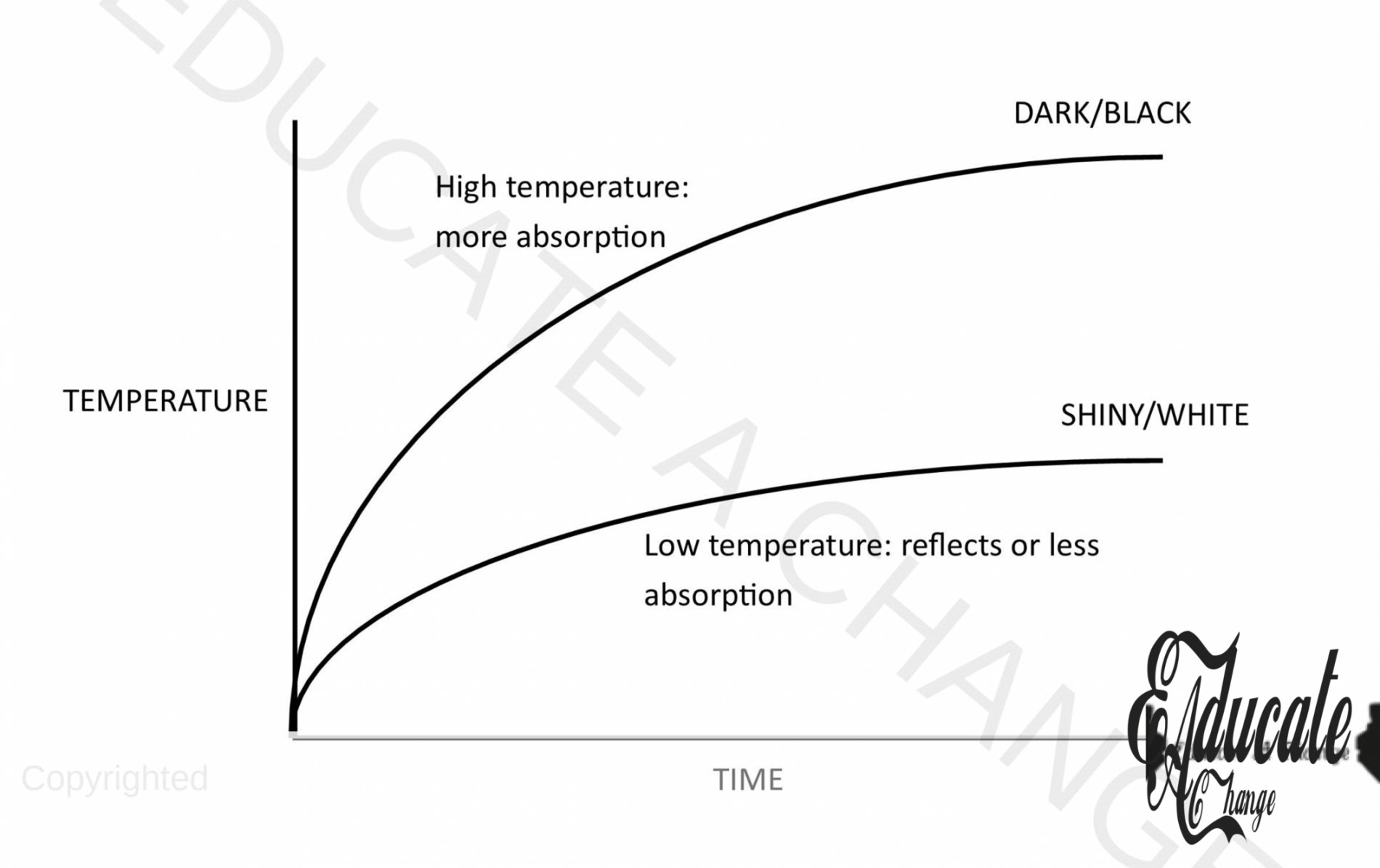
- Notice that the temperature of the black foil sensor is recorded higher than that of the shiny foil.
Conclusion:
- Black and dull/dark surfaces absorb more rays than the light or shiny surfaces.
Experiment for emitting infrared rays:
Objective: To investigate the emission of infrared rays. Apparatus: Two temperature sensors, dataloggers, two identical tin cans (one black, one white), boiling water. Procedure:
- Attach the temperature sensors to the datalogger and insert the sensors into the tins.
- Pour boiling water in tins,
- Let the water cool,
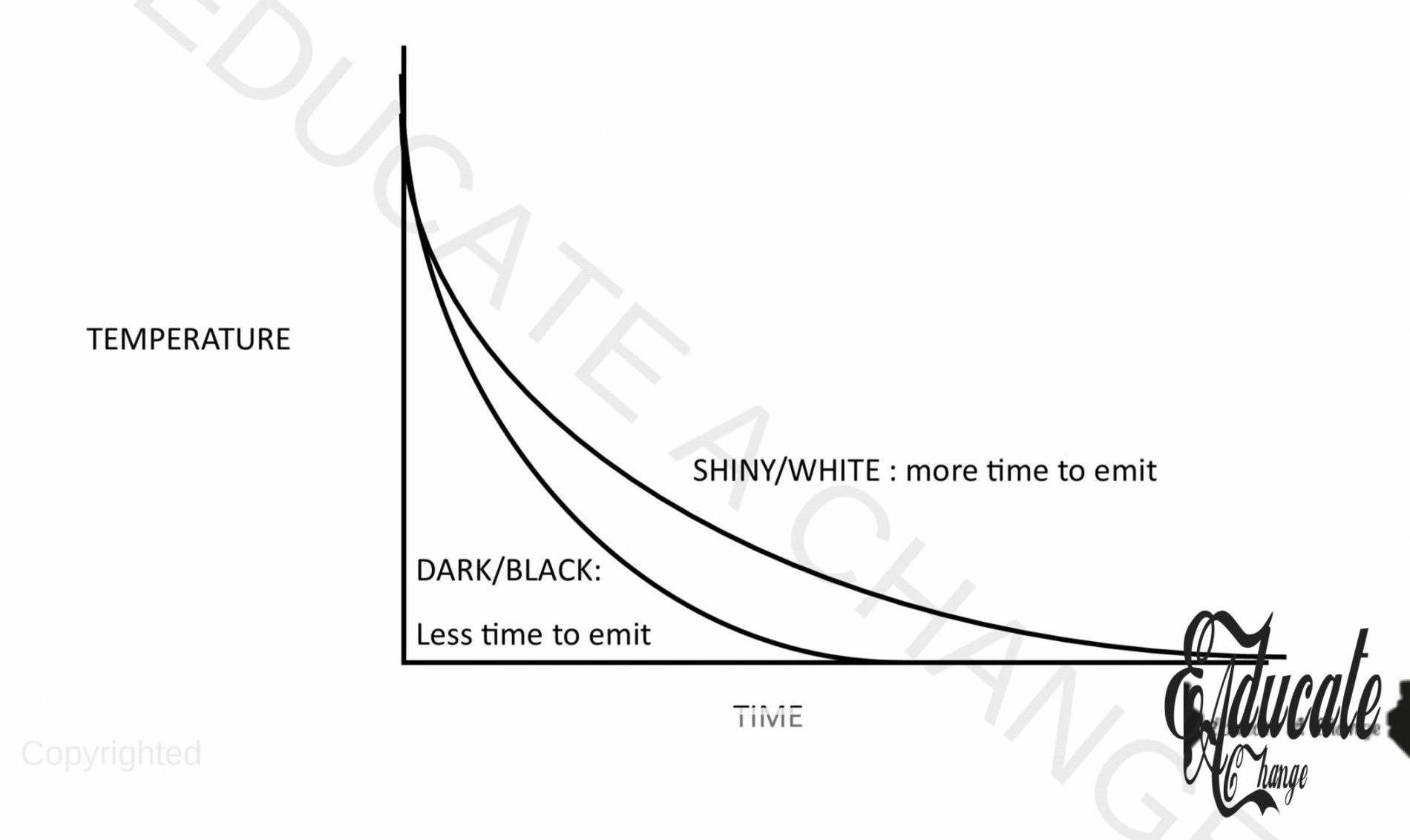
- Notice that the black tin absorbs more heat i.e. the emission of infrared rays is also more. The water in black cools quicker than the white tin
Conclusion:
- Good emitters of radiant heat are also good absorber of heat and vice versa.
Total Transfer
Vacuum Flask:
A vacuum flask is designed in a way to keep a liquid warm. Heat loss is minimized that can occur through conduction, convection, radiation and evaporation.
- Plastic stopper prevents heat loss from the conduction process as plastic is a poor conductor of heat.
- Conduction through trapped air s minimal as air a poor conductor as well.
- Conduction and convection are minimal as there is vacuum in between the double glass.
- T0 minimize the radiation, the walls of the flask are silvered, so it reflects radiant rays.
- This insulation and strategy keep water warm.
Temperature:
Heat and Temperature:
Heat: The amount of thermal energy that flows from hotter object to cooler object. Temperature: It is a measurement of hotness or coolness.
Measuring Temperature:
Properties and Thermometers:
| Physical Property | Thermometer |
| Fixed mass of liquid | Liquid-in-glass thermometer |
| Electromotive force (emf) | Thermocouple |
| Fixed mass of a gas at a constant volume | Pressure-gas thermometer |
Kelvin Scale:
The kelvin scale is designed with the lowest possible temperature to be 0 kelvin. Hence, the lowest possible temperature -273 Celsius is 0 Kelvin. (absolute scale)
Liquid-In-Glass thermometers:
Thermometric Liquids:
There are two mainly preferred thermometric liquids: mercury and alcohol. The following table compared the two liquids.
| Mercury | Alcohol |
| Disadvantages | |
| Poisonous | Can make things wet (form meniscus: where the liquid sticks to the container and makes readings inaccurate) |
| Freezing point -39 | Lower boiling point (78 Celsius) |
| Expensive | Flammable |
| Advantages | |
| Does not evaporate | Maximum freezing point high (-115 Celsius) |
| Cannot wet the tube so no meniscus | Not expensive |
| High boiling point (357 Celsius) | Not poisonous |
Construction of Thermometers:
There are two types of liquid-in-glass thermometers:
| Laboratory | Clinical |
| -10 Celsius to 110 Celsius | 34 to 42 Celsius |
| thermometer is placed in beaker/ fixed | Has a constriction to stop the backward flow of liquid when moved |
| Narrow bore, fine tube | Narrow bore, fine tube, pear shaped so you can see 3 readings from three sides, magnified |
| Thin wall bulb | Thin wall bulb |
| Stem glass is thicker | Stem glass is thicker |
| Liquid with linear expansion | Liquid with linear expansion |
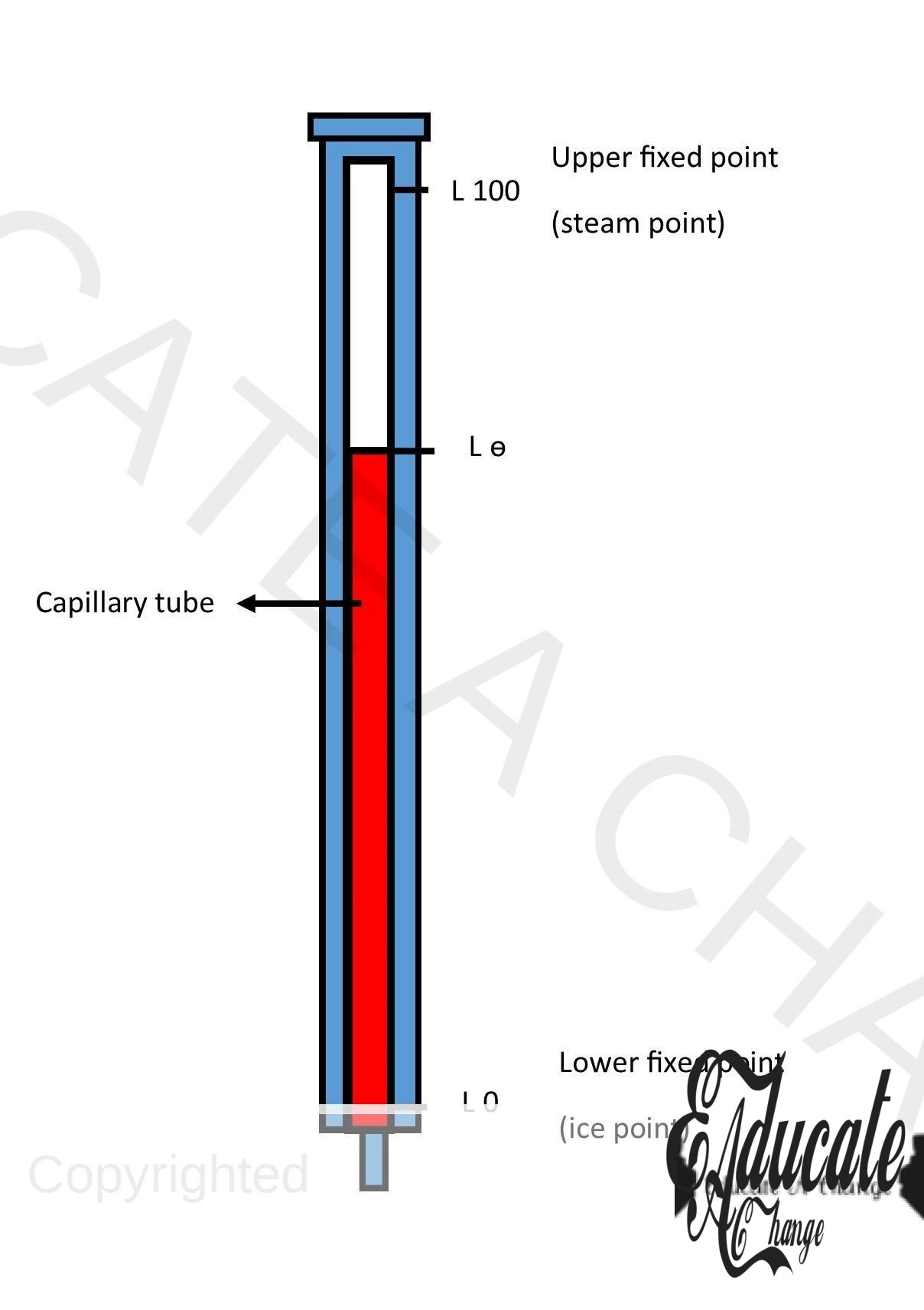
Formula:
- Where l is the value on the thermometer (reading),
- We calibrate the thermometer into 100 equal segments using upper fixed point (steam point) which is the temperature of boiling water and lower fixed point (ice point) which is the temperature of melting ice.
- Each division can be called one degree.
- Depending on what the thermometer measures, l could be length or resistance as well.
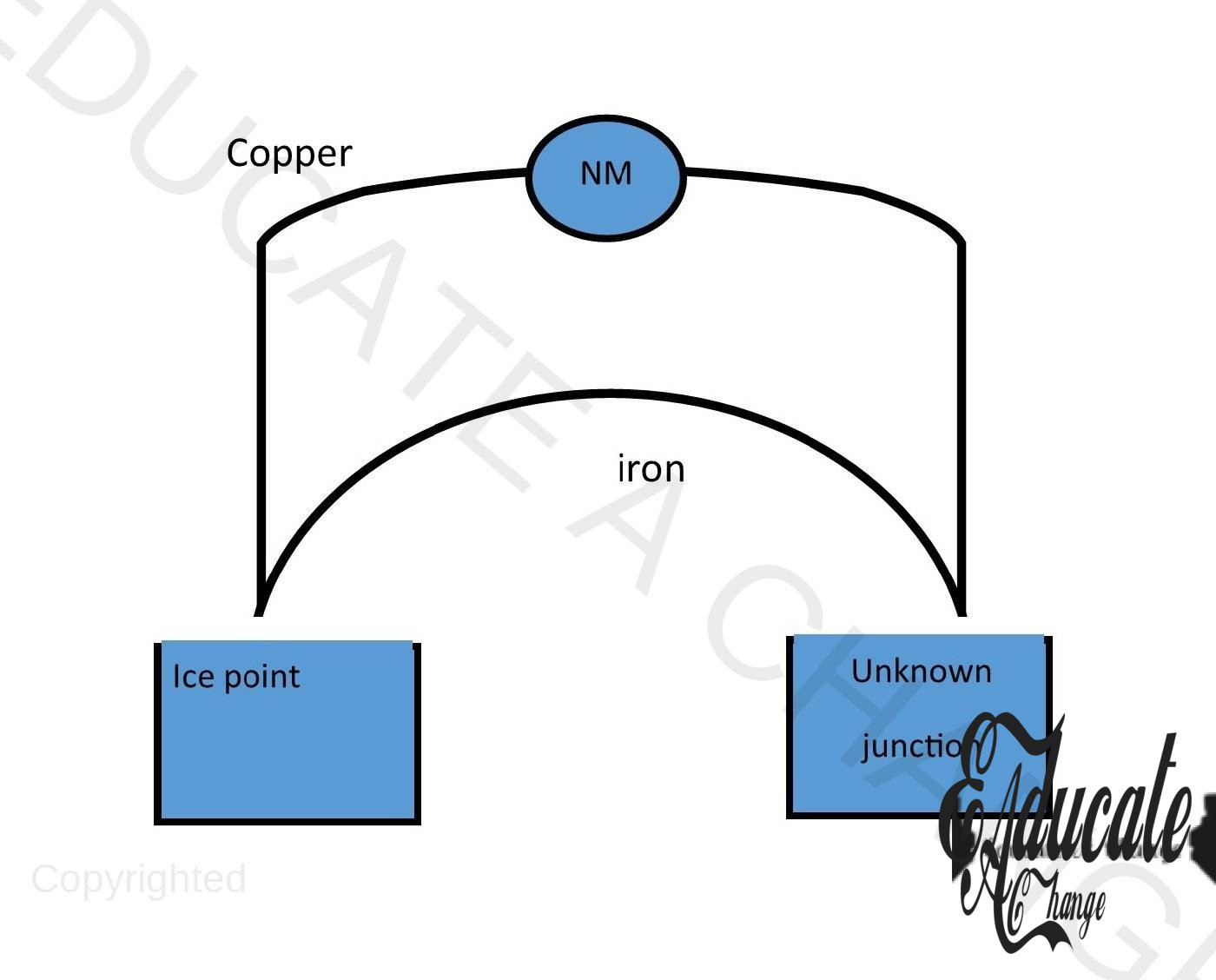
Thermocouple:
- Records temperature,
- Highly accurate,
- High range (-200 Celsius to 1500 Celsius),
- Used where in high temperatures, glass thermometers might not work or melt,
- We fix one of the two junctions of the thermocouple as the ice point and the other is variable.
- There is a copper and iron wire and the difference in their resistivity allows the emf to pass through the device,
- First, we calibrate the thermometer by placing it in boiling water and calibrating it in degrees
- Calibrating means to scale in comparison to a given standard,
- After calibration, we place the other junction in the temperature we need to measure and use our formula to calculate the temperature.
- Emf or electromotive force (discussed in later chapters) is directly proportional to temperature change and hence, it can be used as a temperature measurement.
Range, Sensitivity and Responsiveness:
Sensitivity:
Sensitivity is the rise in mercury column per unit change in temperature.
Responsiveness:
Responsiveness is how quickly the thermometer responds to the change in temperature.
Range:
Range is the minimum and maximum temperature that the thermometer can measure.
Increasing range, sensitivity and responsiveness:
| Range | Sensitivity | Responsiveness |
| Increase the length of the stem | Make the bore narrow | Use a thin wall bulb |
| Increase the diameter of bore | Use a bigger bulb | Use a liquid with better conductivity |
| Use liquid with low expansivity | Use liquid with high expansivity |